Abstract
OBJECTIVE: Hair greying (i.e., canities) is a component of chronological ageing and occurs regardless of gender or ethnicity. Canities is directly linked to the loss of melanin and increase in oxidative stress in the hair follicle and shaft. To promote hair pig-mentation and reduce the hair greying process, an agonist of a-melanocyte-stimulating hormone (a-MSH), a biomimetic peptide (palmitoyl tetrapeptide-20; PTP20) was developed. The aim of this study was to describe the effects of the designed peptide on hair greying.
METHODS: Effect of the PTP20 on the enzymatic activity of cata-lase and the production of H2O2 by Human Follicle Dermal Papilla Cells (HFDPC) was evaluated. Influence of PTP20 on the expression of melanocortin receptor-1 (MC1-R) and the production of melanin were investigated. Enzymatic activity of sirtuin 1 (SIRT1) after treatment with PTP20 was also determined. Ex vivo studies using human micro-dissected hairs allowed to visualize the effect of PTP20 on the expression in hair follicle of catalase, TRP-1, TRP-2, Melan-A, ASIP, and MC1-R. These investigations were completed by a clinical study on 15 human male volunteers suffering from premature canities.
RESULTS: The in vitro and ex vivo studies revealed the capacity of the examined PTP20 peptide to enhance the expression of catalase and to decrease (30%) the intracellular level of H2O2. Moreover, PTP20 was shown to activate in vitro and ex vivo the melanogene-sis process. In fact, an increase in the production of melanin was shown to be correlated with elevated expression of MC1-R, TRP-1, and Melan-A, and with the reduction in ASIP expression. A modu-lation on TRP-2 was also observed. The pivotal role of MC1-R was confirmed on protein expression analysed on volunteer’s plucked hairs after 3 months of the daily application of lotion containing 10 ppm of PTP20 peptide.
CONCLUSION: The current findings demonstrate the ability of the biomimetic PTP20 peptide to preserve the function of follicular melanocytes. The present results suggest potential cosmetic application of this newly designed agonist of a-MSH to promote hair pigmentation and thus, reduce the hair greying process.
Resume
OBJECTIF: Le blanchiment des cheveux (i.e., canitie) est un element du vieillissement chronologique qui se produit independamment du genre ou de l’ethnie. La canitie est directement liee a la perte de melanine et a l’augmentation du stress oxydatif dans le follicule et la tige pilaires. Pour favoriser la pigmentation des che-veux et reduire le processus de blanchiment des cheveux, un ago-niste de l’« a-melanocyte-stimulating hormone » (a-MSH), un peptide biomimetique (« palmitoyl tetrapeptide-20 »; PTP20) a ete developpe. Le but de cette etude est de decrire les effets de ce peptide concu, sur le blanchiment des cheveux.
METHODES: L’effet du PTP20 a ete evalue sur l’activite enzymatique de la catalase et la production de H2O2 par les cellules humaines de la papille dermique du follicule pileux (« Human Folli-cle Dermal Papilla Cells », HFDPC). L’influence du PTP20 sur l’expression du recepteur alamelanocortine (« melanocortin recep-tor-1 », MC1-R) et sur la production de melanine ont ete etudies. L’activite enzymatique de la sirtuine 1 (SIRT1), apres traitement avec PTP20, a aussi etedeterminee. Des etudes ex vivo sur cheveux humains microdisseques ont permis de visualiser l’effet du PTP20 sur l’expression de la catalase, TRP-1, TRP-2, Melan-A, ASIP et MC1-R, dans le follicule pileux. Ces etudes ont ete completees par un essai clinique incluant 15 volontaires masculins souffrant de canitie precoce.
RESULTATS: Les etudes in vitro et ex vivo ont revele la capacitedu peptide PTP20 examine, a augmenter l’expression de la catalase et a diminuer (30%) le niveau intracellulaire de H2O2. De plus, il a ete montre que PTP20 activait le processus de melanogenese in vitro et ex vivo. En fait, il a ete montre qu’une augmentation de la production de melanine etait correlee avec une expression plus elevee de MC1-R, TRP-1 et Melan-A, ainsi qu’avec une reduction de l’expression d’ASIP. Une modulation de TRP-2 a aussi ete observee. Le r^ole cle de MC1-R a ete confirme apres analyse d’expressions proteiques, sur les cheveux epiles des volontaires, apres 3 mois d’application quotidienne d’une lotion contenant 10 ppm du peptide PTP20.
CONCLUSION: Les conclusions actuelles demontrent la capacite du peptide biomimetique PTP20 apreserver la fonction des melanocytes folliculaires. Les resultats presentes suggerent une potentielle application cosmetique de cet agoniste d’ a-MSH nouvellement concu, pour favoriser la pigmen-tation et par consequent, reduire le processus de blanchimentdes cheveux.
Introduction
Hair colour is due to the presence of a pigment, melanin, pro-duced by melanocytes and transferred to keratinocytes. In fact, the hair follicle pigmentation results from precise sequential interac-tions between follicular melanocytes, matrix keratinocytes, and dermal papilla fibroblasts. It involves successively the melanogenic activity of follicular melanocytes, the transfer of their product, melanin granules, into cortical and medullary keratinocytes, and the formation of pigmented hair shafts.
Numerous mechanisms, controlled by a great number of various protein factors, acting at different stages of follicular melanogenesis, contribute to hair greying, including the loss of melanocyte stem cells or their failure to differentiate, melanocyte migration defects, melanocyte apoptosis, and pigmentary machinery malfunction. Indeed, as reported previously, the appearance of white hair is a phenomenon directly associated with a decrease in follicular mela-nocyte population and a decrease in melanin content. A buildup of reactive oxygen species with a decreased ability to handle oxida-tive damage is also implicated in the process of hair greying.
The recently proven concept of H2O2-induced oxidative damage in the entire hair follicle, including the hair shaft, is considered as one of key elements in senile hair greying. As reported by Wood et al., human grey/white scalp hair shafts accumulate hydrogen peroxide (H2O2) in millimolar concentrations. In fact, FT-Raman spectra showed in vivo the presence of 10-3 mol/L H2O2 concentra-tions in grey and completely white hair.
It is well known that catalase, one major degrading enzyme for H2O2, constitutes the body’s primary defense against hydrogen per-oxide damage. However, recent investigations revealed that catalase protein expression and hydroxyl radical scavenging activities are strongly repressed in unpigmented hair follicles leading consequently to accumulation of hydrogen peroxide, which is a chemical com-pound with strong oxidizing properties. In addition to its direct bleaching effect (oxidation of endogenous melanin), H2O2 also inhi-bits the synthesis of melanin. Indeed, the structural modification of tyrosinase due to the oxidation of its active site Met 374 limits the functionality of this key enzyme in melanogenesis, which leads to gradual loss of hair colour. In this context, it is noteworthy that oxidation and deactivation of catalase by its own substrate, hydrogen peroxide, also occurs. H2O2-mediated oxidation resulting in the loss of biological activity has been also documented for many other impor-tant regulators of pigmentation, including the pro-eumelanogenic pep-tide a-melanocyte-stimulating hormone (a-MSH).
It is well-documented that a-MSH and its receptor MC1-R (melanocortin 1 receptor) are the key regulators of melanin pigment generation in both skin and hair. Thus, each structural and functional perturbation of this hormone or/and its receptor will result in the decline in hair pigmentation.
The effects of a-MSH are mediated by binding to MC1-R, expressed preferentially on normal human melanocytes and recog-nized as a key-signalling molecule of cutaneous melanogenesis. In fact, it plays a pivotal role in the activation of downstream factors, followed by sequential activation of MITF (microphthalmia-asso-ciated transcription factor), which regulates the expression of tyrosinase, TRP-1 (tyrosinase related protein 1) and TRP-2 (tyrosi-nase related protein 2).
It has been shown that MC1-R signalling is negatively regulated by an endogenous ligand ASIP (agouti signalling protein). ASIP blocks the stimulatory effect of a-MSH on tyrosinase and decreases TRP-1 and TRP-2 gene expression and total melanin production. Although ASIP’s primary sequence has no similarities to a-MSH, it binds to MC1-R with almost equal affinity. Thus, the secreted ASIP protein could affect the quality of hair pigmentation. Indeed, polymorphisms in ASIP are associated with skin, hair, and eye pigmentation phenotypic changes.
Emerging evidence suggests also a role of sirtuin 1 (SIRT1) in maintaining capillary homeostasis. This nuclear protein belonging to the family of sirtuins plays a key role in the control of the ageing process reducing the incidence of age-related disorders. It was reported that animals characterized by reduced levels of SIRT1 had a phenotype of accelerated ageing and exhibited several features of premature skin and hair ageing. Thus, the enhanced expression of SIRT1 could antagonize hair pigmentation age-related decline.
he constantly growing knowledge concerning hair follicle biology and the causes of hair depigmentation open new strategies for intervention and reversal of the hair greying process. To fight against can-ities we developed an agonist of a-MSH, a biomimetic peptide (palmitoyl tetrapeptide-20; PTP20). In the current paper, we describe the effects of this peptide on different molecular targets related to hair greying and we provide the results of clinical studies on the anti-grey-ing efficacy of a topical formulation containing this bioactive peptide.
Material and methods
Peptide design
Palmitoyl tetrapeptide-20 synthesis
Cell culture
Evaluation of the MC1-R receptor transactivation
When the plasmid construct was activated by PTP20, there was a luminescent signal measured and quantified by a lumi-nometer. The AC50 was determined. It corresponds to the concen-tration which activates 50% of the luminescence compared to control.
Evaluation of melanin synthesis
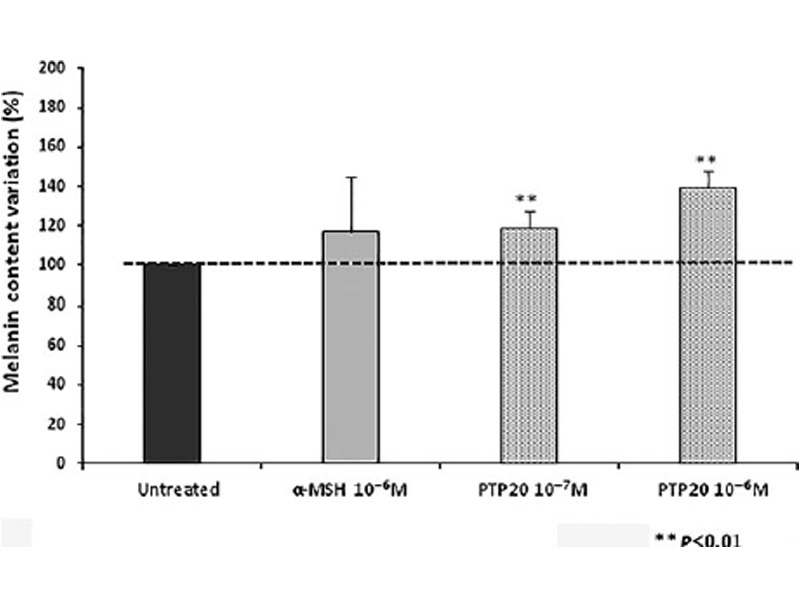
HEMa cells have been seeded in 6-well plates at the concentration of 3.105 cells/well. After 24 h, the medium was removed and the cells were treated with PTP20 (10-6 and 10-7 M) or a-MSH (10-6 M). After 72 h of treatment, cells were detached and incu-bated 10 min at 100°C, with NaOH at 1M, for melanin extraction. Activity of tested products relatively to a-MSH activation was eval-uated by spectrophotometry at λ = 405 nm. Melanin content is expressed as the percentage of melanin production, compared to cell number. Experiments were performed in triplicate.
Determination of catalase enzymatic activity
The effect of PTP20 on the enzymatic activity of catalase was also investigated by evaluation of intracellular levels of H2O2 using a fluorescent dichlorofluorescein assay adapted to flow cytometry which allows detection of picomole levels of hydroperoxides.
Using the fluorogenic probe, the 20,70-dichlorofluorescin diacetate (DCFH-DA) from Sigma (Saint-Quentin Fallavier, France), we assessed the levels of intracellular hydrogen peroxide within the control and treated cells. Briefly, HFDPC cells were seeded at 50 000 cells/well in 12-well plates with 500 μL of complete med-ium and incubated for 24 h at 37°C. PTP20 (10-5 M final concen-tration) was then added and incubated for 18 h at 37°C. At the end of incubation, DCFH-DA probe (10 μM final concentration) was added for additive 15 min. Cells were then trypsinized, washed, and immediately scanned on FACS (FC500 flow cytometer from Beckman-Coulter, Villepinte, France) with excitation and emission settings of 485 and 529 nm, respectively. The emitted fluorescence is assumed to be proportional to the concentration of hydrogen peroxide in the cells. The results are expressed as percentage of decrease in the H2O2 production by HFDPC, in com-parison with the untreated cells.
SIRT1 activity evaluation
After 30 min of incubation, the enzymatic reaction was stopped and the amount of remaining acetylated substrate was assessed using a specific antibody coupled to a fluorophore. The quantifica-tion of emitted fluorescence allows to measure the activity of SIRT1. The results are expressed as percentage of increase in SIRT1 activity in comparison to the control values obtained without addi-tion of the tested peptide.
Microdissected human hairs ex vivo
Eleven hairs were treated with the product PTP20 added to the culture medium at 10-9 M on day 0, day 1, day 4, and day 6 while the culture media of the 11 untreated control hairs were completely renewed on the same days.
On day 0 and day 7, the hairs were harvested for histological analysis. For each condition, half of the hairs were fixed in standard formalin-buffered solution for 24 h, dehydrated and embedded in paraffin; the other half were frozen at -80°C for immunostainings.
In vivo clinical study
Fifteen healthy Caucasian male volunteers were included in a clinical study after informed consent. They were between 18- and 38 year-old (average age 33 + 5 years) and exhibited a premature hair greying, with more than 20% of white hairs.
Formulation
The active peptide PTP20 was added at 10 ppm in the following colorless lotion, using the described formulation process:
| Phase | Ingredient | Supplier | % |
| A | DEIONIZED WATER | 78.51 | |
| B1 | ALCOHOL | CHARBONNEAUX – BRABANT | 14.73 |
| B1 | PROPYLENE GLYCOL | INTERCHIMIE | 2 |
| B1 | LECITHIN | LUCAS MEYER | 1 |
| B2 | 500 ppm PTP20 SOLUTION | LUCAS MEYER | 2 |
| C | PEG-40 HYDROGENATED CASTOR OIL |
SAFIC-ALCAN | 1.5 |
| D1 | PVP | ASHILAND | 0.15 |
| D3 | PANTHENOL | BASF | 0.1 |
- In a container solubilize lecithin in propylene glycol and in alcohol at room temperature with gentle agitation. The solution must be clear and homogeneous.
- Add the peptide solution at room temperature with gentle stirring. The solution must be homogeneous.
- Add phase B to phase A at room temperature with medium agitation.
- Pre-mix phase C in an auxiliary container, with gentle stirring, until complete solubilization. The mixture should be clear and homogeneous.
- Then add phase C to the manufacturing vessel at 30°C with medium agitation. Thoroughly homogenize the solution.
- Then add the ingredients of phase D, one by one, to the main container, with medium agitation. Check the homogeneity of the solution between each addition. Thoroughly homogenize the solution.
The described above lotion was applied topically on dry hair scalp, at the dose of 3 mL dispensed into five pipettes with a massage to facilitate the hair scalp distribution, without rinse. The application was repeated every day for 3 months (from Mai to July), except the last day of sampling.
Plucked hairs
On day 0, and after 3 months treatment, several hairs were plucked from each volunteer hair scalp. For each volunteer, half of the plucked hairs were fixed in standard formalin-buffered solution for 24 h, dehydrated and embedded in paraffin, and half were frozen at -80°C for immunostainings.
Stainings & fluorescent immunostainings & immunohistochemistry
For fluorescent immunostainings, the primary antibodies used were anti-PARD3/ASIP rabbit antibody (Abcam, ref. ab64646) diluted at 1/200 for 1 h at RT and anti-catalase goat antibody (Santa-Cruz biotechnologies, ref. sc-34280) diluted at 1/100 over-night at 4°C. The sections were then incubated for 30 min at room temperature with Alexa Fluor 488-labelled rabbit anti-goat antibody (Lifetechnologies, ref. A11078) or AF488-labelled goat anti-rabbit antibody (Lifetechnologies, ref. A11008) diluted at 1/ 1000 in PBS with 3% BSA. The nuclei were counterstained using propidium iodide at 0.2 lg/mL for 2 min. The sections were finally mounted using Vectashield® mounting medium (Vector, ref. H-1400) or Fluoromount-G® mounting medium (Southern Biotech, cat n°0100-01).
For Immunohistochemistry, the hair sections were pre-incubated with hydrogen peroxide (VWR, ref. 23619.264) at 0.3% in PBS for 10 min to inactivate endogenous peroxidase activity. The primary antibodies used were anti-TRP-1 mouse antibody (Eurogentec, ref. SIG-38150-1000) diluted at 1/200 for 1 h at RT, anti-MC1-R rabbit antibody (Santa Cruz biotechnologies, ref. sc-28990) diluted at 1/200 for 1 h at RT, anti-Melan-A mouse antibody (Santa Cruz biotechnologies, ref. sc-20032) diluted at 1/100 for 1 h at RT and anti-TRP-2 mouse anti-body (Santa Cruz biotechnologies, ref. sc-74439) diluted at 1/500 for 1 h at RT. The sections were incubated for 30 min at room tempera-ture with a horse anti-mouse/rabbit biotinylated secondary antibody, then incubated for 30 min at room temperature with a streptavidin-labelled peroxidase (Vector, Vectastain® Universal ABC kit, ref. PK7200). The staining was revealed by a violet substrate of peroxi-dase, VIP (Vector, ref. SK-4600) from 2 to 4 min. The nuclei could be counterstained using Mayer hemalun (RAL diagnostics, ref. 320550). The sections were finally dehydrated and mounted using Eukitt mounting medium (VWR, ref. KIND01250).
The microscopical observations were realized using a Leica DMLB or Olympus BX43 microscope. Pictures were digitized with a numeric DP72 Olympus camera with CellD storing software.
Statistical analysis
Results
Effect of PTP20 on MC1-R receptor transactivation
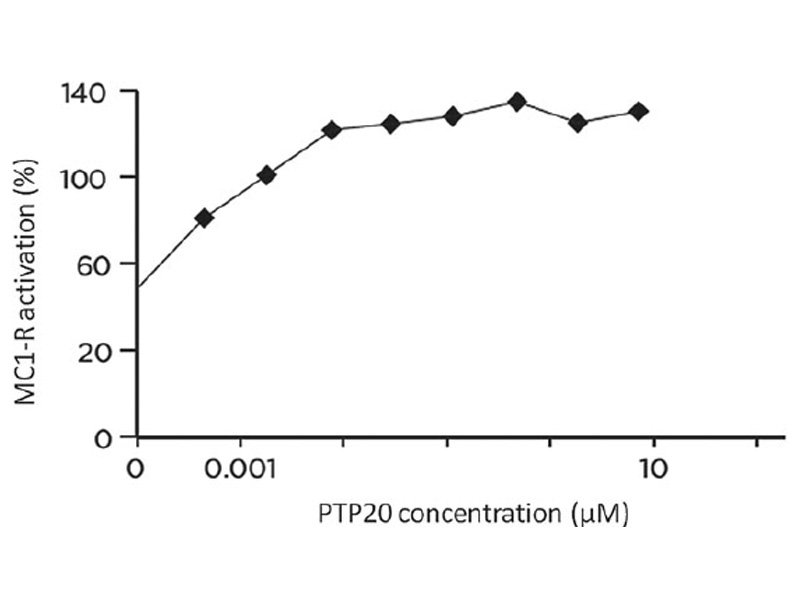
The ability of the peptide to modulate the transactivation of MC1-R was evaluated after co-transfection of HEK293 cells. The dose-response of MC1-R receptor activation by the PTP20 is presented in Fig. 1. The maximum activation (137%) was obtained by 10 µM of peptide with an AC50 = 0.16 nM. The lower is the AC50, the better is the affinity of the molecule for its receptor.
Effect of PTP20 on melanin production
Figure 1
MC1-R activation by PTP20. The dose-response of PTP20 was tested on the transactivation studies in HEK293-hMC1-R.
Figure 2
PTP20 peptide increases melanin synthesis in comparison to the untreated control (**P < 0.01).
Effect of PTP20 on the enzymatic activity of catalase
Two different experimental approaches were used to evaluate the catalase activity. A preliminary assessment was done with the com-mercial kit ‘Amplex Red Catalase Assay’ and allowed to demonstrate an increase of 10% in the enzymatic activity of cata-lase incubated with a 10-5 M PTP20 solution.
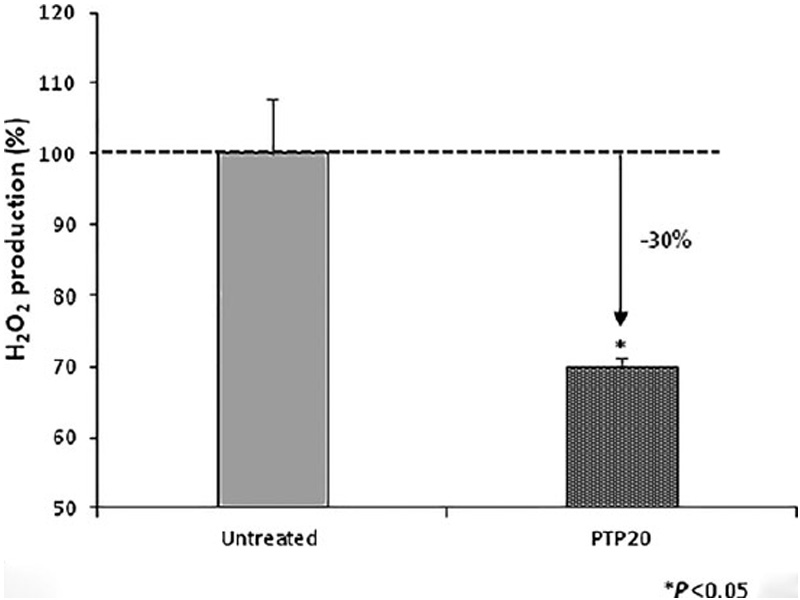
The effect of PTP20 peptide on the enzymatic activity of catalase investigated by evaluation of intracellular levels of H2O2, using a fluorescent assay adapted to flow cytometry, stays in line with earlier finding. Indeed, the incubation of hair follicle dermal papilla cells for 18 h with PTP20 peptide resulted in a significant decrease of 30% in the intracellular level of H2O2 (Fig. 3).
Effect of PTP20 peptide on the enzymatic activity of SIRT1
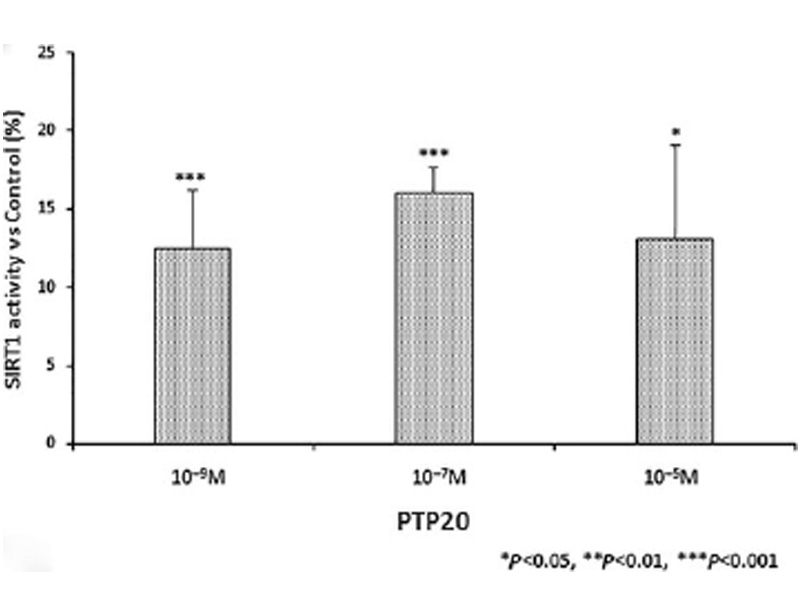
A high-throughput HTRF SIRT1 assay was used to study the impact of PTP20 on the activity of SIRT1. The obtained results (Fig. 4) showed that the treatment of purified SIRT1 with PTP20 stimulated SIRT1 activity. Indeed, a significant increase from 12.5% to 16% in the measured activity was noted for PTP20 tested from 10-9 M to 10-5 M concentration, respectively.
Effect of PTP20 peptide on human hairs ex vivo
Table I
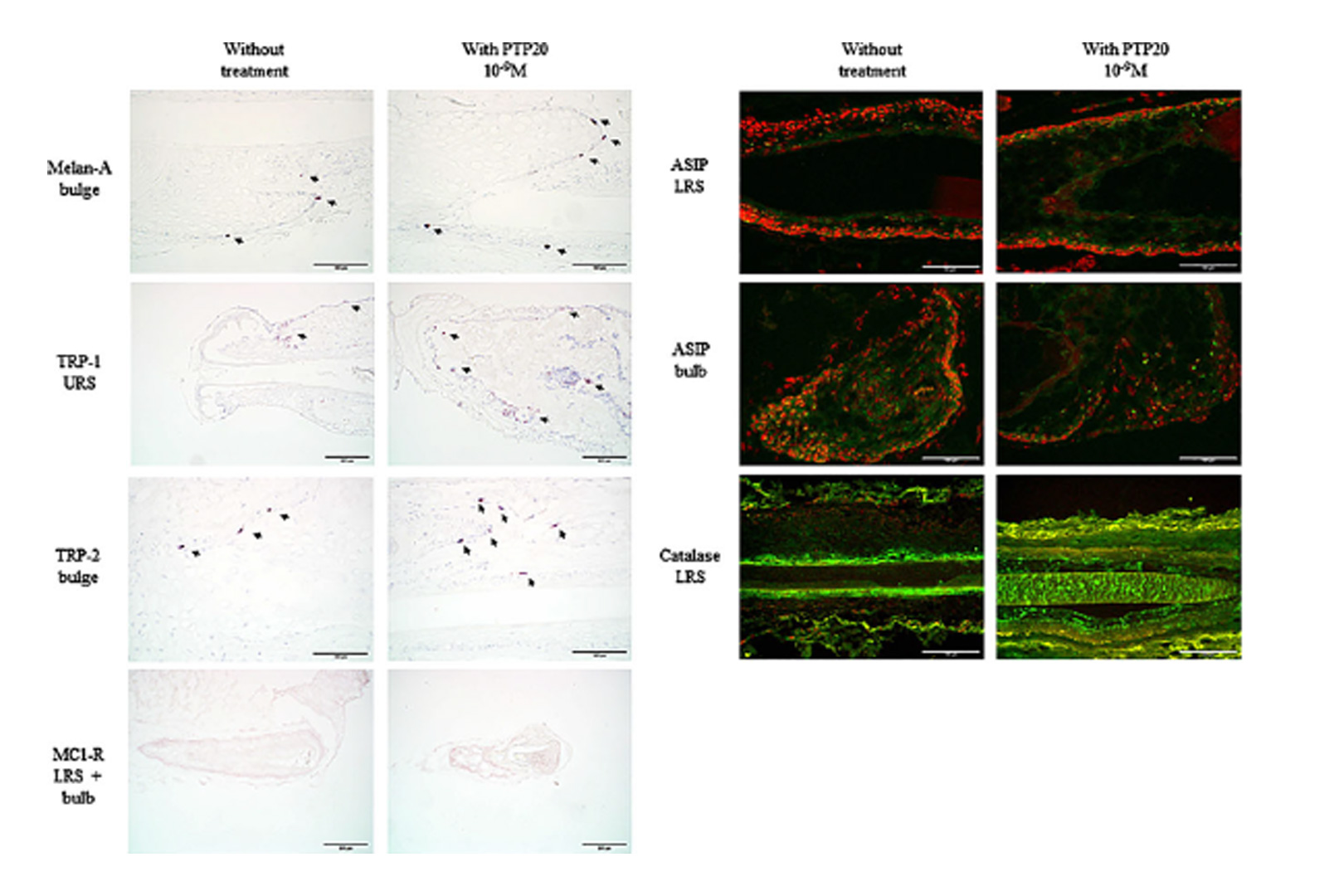
Immunostaining variation (intensity and frequency) after PTP20 ex vivo treatment of human hair follicles
| Markers with variation of staining vs untreated hairs | 10-9 M PTP20 after 7 days |
| Melan-A | + bulge |
| TRP-1 | + upper root sheaths |
| TRP-2 | + bulge |
| MC1-R | + lower root sheaths
+ bulb |
| ASIP (PARD3) | – lower root sheaths |
| (weak detection) | – bulb |
| Catalase | + lower root sheaths |
Figure 5 Immunostaining of Melan-A, TRP-1, TRP-2, (see arrows) and MC1-R (in violet) and immunostaining of ASIP/PARD3 and catalase (in green with nuclei in red) on ex vivo control or treated for 7 days with PTP20 human hair follicles. URS: upper root sheaths; LRS: lower root sheaths. Scale bar 100 lm (Melan-A, TRP-2, ASIP/PARD3, and catalase); scale bar 200 lm (TRP-1 and MC1-R).
Was slightly increased especially in the bulge area, a location of melanocyte stem cells. The expression of a-MSH receptor, MC1-R, was also slightly increased whereas the expression of the Agouti signalling protein, ASIP/PARD3, was slightly decreased in the lower part of the hair follicle (lower root sheaths and bulb). Two other melanosomal proteins, tyrosinase-related proteins TRP-1 and TRP-2, were slightly induced in the upper root sheaths and the bulge, respectively. The anti-oxidant enzyme catalase, known to block the deleterious whitening effect of hydrogen peroxide in hair, was moderately increased in the lower root sheaths (Table I & Fig. 5).
Effect of PTP20 lotion observed during in vivo clinical study
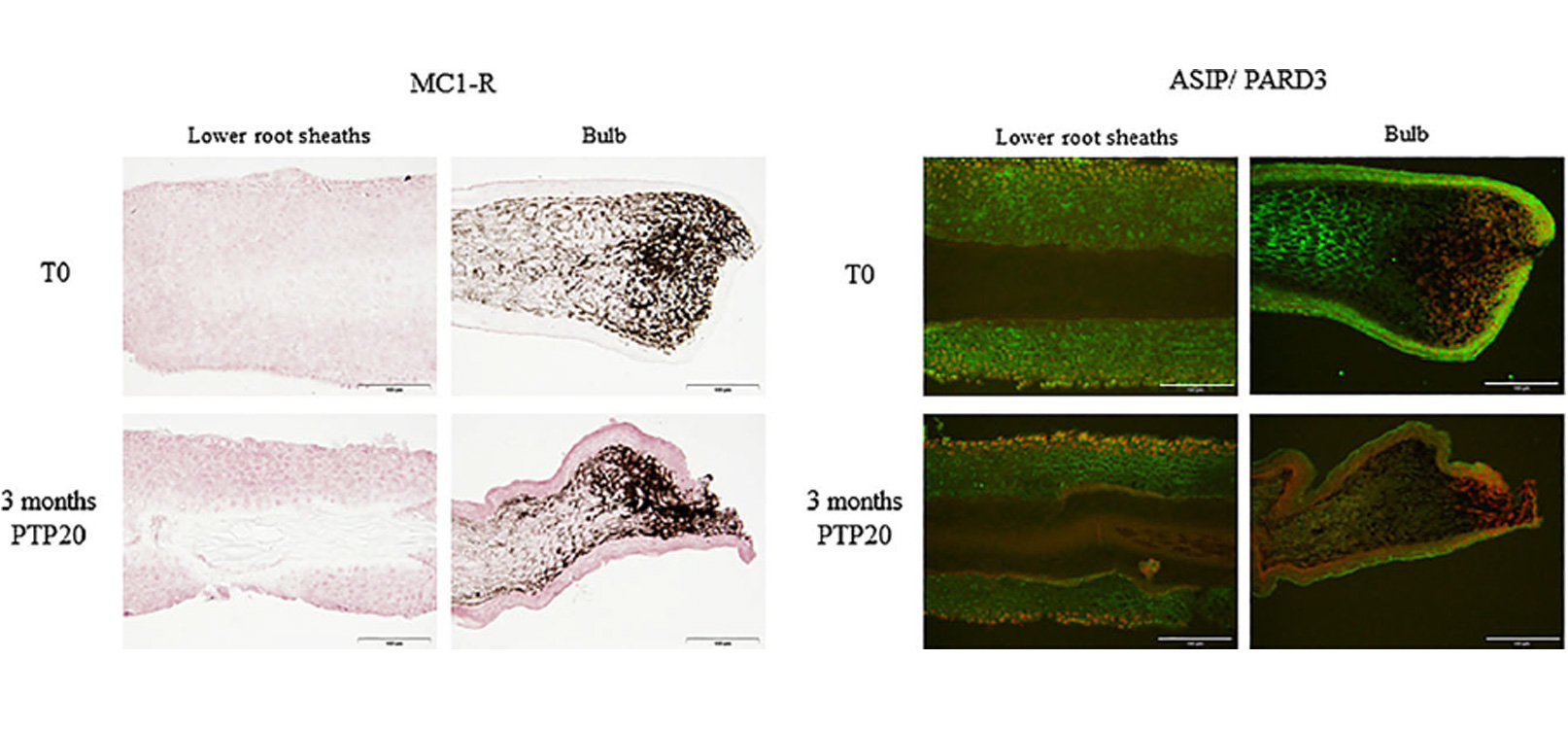
Table II Immunostaining variation (intensity and frequency) of MC1-R and ASIP/PARD3 on plucked hairs after 3 months in vivo treatment with PTP20 lotion (n = 15), compared to T0
| Markers with variation of staining vs T0 | PTP20 active lotion after 3 months |
| MC1-R | + lower root sheaths (67% of volunteers)
+ bulb (53% of volunteers) |
| ASIP (PARD3) | – lower root sheaths (62% of volunteers)
– bulb (64% of volunteers) |
Discussion
Thus, taking into account the major role of a-melanocyte-stimu-lating hormone (a-MSH) in the hormonal regulation of melanogenesis, we developed an agonist of a-MSH, a biomimetic peptide (palmitoyl tetrapeptide-20, PTP20). Our in vitro, ex-vivo, and in vivo investigations revealed a great number of biological activities of the studied peptide, which contribute to the process of hair pigmentation. Indeed, the results of in vitro studies confirmed the MC1-R agonist property of PTP20 peptide and revealed its capacity to increase significantly melanin synthesis in cultured human melanocytes. Moreover, MC1-R expression was up-regulated by PTP20 in ex vivo experiments as well as in clinical studies.
It is well known that besides a-MSH, its receptor MC1-R and tyrosinase are the major targets in the medicinal/cosmetic hair greying treatment. It has been previously shown that mice with knockout of the MC1-R had premature greying of their fur. Furthermore, recent reports on the potency of natural root extract to induce melanin synthesis point out the activation of MC1-R/MITF/tyrosinase- signalling pathway and an increase in MC1-R expression as key elements of observed promotion of hair pigmentation. Moreover, it was found that early hair greying phenomenon may be related to downregulation of this pathway [19]. The activation of MC1-R pathway can be inhibited by agouti signalling protein (ASIP). Indeed, ASIP is an important negative regulator of hair pigmentation as it competitively prevents the binding of a-MSH to MC1-R and inhibits melanogenesis. Considering the pivotal role of MC1-R in inducing expression of melanogenic enzymes, the melanin level increase reported here, together with the stimulation of MC1-R, TRP-1, and TRP-2 expression, is consistent with the observed down-regulation of ASIP expression in the human follicle following PTP20 treatment ex vivo and in vivo.
Moreover, the agouti gene encodes a paracrine signalling molecule that causes hair follicle melanocytes to synthesize the yellow pigment pheomelanin instead of the black or brown pigment eumelanin. In addition, TRP-2 converts the melanogenic intermediate DOPAchrome to DHICA (5,6-dihydroxyindole-2-carboxylic acid), therefore affecting eumelanin, but not pheomelanin synthesis. TRP-2 is downregulated during pheomelanin synthesis. Ex vivo and clinical data showing the decreasing effect of PTP20 on ASIP expression and its increasing effect on TRP-2 expression, suggested the potential efficacy of PTP20 to repigment the grey hair with brown pigments.
The physiological hair greying that occurs with ageing is also known to result, in part, from accumulation of oxidative damage generated during normal metabolism. The growing number of data provided the clear evidence of primordial role of catalase deficiency and consequent hydrogen peroxide accumulation in the phenomenon of hair greying.
In vivo identification of massive H2O2 concentrations in the grey hair shaft introduced a new step in the understanding of human hair greying at the biochemical and molecular level. Depigmentation of the hair is in fact related to failure of the action of tyrosinase and a-MSH, structurally damaged and functionally altered by H2O2-mediated oxidation as well as to direct oxidation of melanin leading to the pigment degradation (bleaching effect). This advanced insight opens new strategies for intervention and reversal of the hair greying process. It might be expected that enhanced expression/activity of catalase could result in the reduction of canities. One should add that a particular target of research on canities is the nature of the melanocyte stem compartment and a failure of melanocyte stem cell renewal due to oxidative stress. Taking into consideration the reported here abilities of PTP20 peptide to enhance catalase expression and reduce the intracellular level of H2O2 it is tempting to speculate on the ability of PTP20 to also protect melanocytes stem cell population against oxidative damage.
It is also worth emphasizing the role of growth factors as key regulators of hair follicle homeostasis and melanogenesis. Indeed, it has been shown that Stem Cell Factor (SCF) expression positively correlates with the rise of tyrosinase activity. Moreover, SCF is crucial for melanocyte survival during development, and its gene mutation results in unpigmented hairs. Hepatocyte Growth Factor (HGF) is known to promote in vivo survival, proliferation and differentiation of melanocyte precursors and its expression prevents hair greying. It was also reported that Platelet Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF) both activate melanocyte stem cells, and are efficient for reversal of hair greying. Finally, Nerve Growth Factor (NGF) was found to rescue melanocytes from apoptosis. The results of preliminary one shoot experiment carried out in vitro with HFDPC cells revealed that the cell treatment with 10-9 M to 10-5 M PTP20 enhanced to a different degree (17–90%) the pro-duction of all these growth factors. These findings corroborate the anti-greying effect of PTP20. However, complementary studies are needed to precise the individual effect of PTP20 on the expression and production of each of these factors.
An age-related hair pigment loss becomes the inescapable signal of our disappearing youth. Over the past decade, a large number of regulators and signalling pathways have been identified for the control of ageing and longevity. They include among others SIRT1, a nuclear enzyme belonging to the family of sirtuins and recognized as a longevity protein and key regulator of cell survival in response to stress. The bibliographic data clearly indicate that the SIRT1 protein prolongs the life of fibroblasts and protects keratinocytes against UVB and H2O2-induced cell death. The data from in vitro experiments support the idea that SIRT1 activity is critically required for melanocyte cell proliferation. Indeed, SIRT1 suppression induced a senescence-like phenotype and its associated cell proliferation arrest of human melanoma cells. Thus, the activation of SIRT1 by PTP20 could have an anti-ageing effect favoring the survival of both melanocytes and cells forming the hair follicle and exhibiting a potential protective effect against hair greying.
Conclusions
The purpose of this study was to evaluate the effects of a new agonist of a-MSH, the tetrapeptide PTP20, on hair greying. The findings presented in this paper provide several concordant lines of evidence, in vitro, ex vivo, and in vivo in support of biological efficacy of this peptide to stimulate the process of hair pigmentation. In fact, the data we are reporting might yield clues to possible therapies for the prevention and/or the reduction of hair greying process and strongly suggest potential cosmetic applications of this new mime of a-MSH.
Acknowledgements
REFERENCES
- Panhard, S., Lozano, I. and Loussouarn, G. Greying of the human hair: a worldwide survey, revisiting the ‘50’ rule of thumb. Br. J. Dermatol. 167(4), 865–873 (2012).
- Slominski, A., Wortsman, J., Plonka, P.M., Schallreuter, K.U., Paus, R. and Tobin, D.J. Hair follicle pigmentation. Review. J Invest Dermatol. 124(1), 13–21 (2005).
- Tobin, D.J. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res. 24(1), 75–88 (2011).
- Sarin, K.Y. and Artandi, S.E. Aging, graying and loss of melanocyte stem cells. Stem Cell Rev. 3, 212–217 (2007).
- Arck, P.C., Overall, R., Spatz, K. et al. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 20(9), 1567–1569 (2006).
- Wood, J.M., Decker, H., Hartmann, H. et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 23(7), 2065–2075 (2009).
- Spencer, J.D., Gibbons, N.C., Rokos, H., Peters, E.M., Wood, J.M. and Schallreuter, K.U. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J Invest Dermatol. 127(2), 411–420 (2007).
- Gibbons, N.C.J., Wood, J.M., Rokos, H. and Schallreuter, K.U. Computer simulation of native epidermal enzyme structures in the presence and absence of hydrogen peroxide (H2O2): potential and pitfalls. J. Invest. Dermatol. 126, 2576–2582 (2006).
- Dos Videira, I.F. S, Moura DFL, Magina S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 88(1), 76–83 (2013).
- Rodrigues, A.R., Almeida, H. and Gouveia, A.M. Intracellular signaling mechanisms of the melanocortin receptors: current state of the art. Review. Cell Mol Life Sci. 72(7), 1331–1345 (2015).
- Suzuki, I., Tada, A., Ollmann, M.M. et al. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. J Invest Dermatol. 108(6), 838–842 (1997).
- Siegrist, W., Willard, D.H., Wilkison, W.O. and Eberle, A.N. Agouti protein inhibits growth of B16 melanoma cells in vitro by acting through melanocortin receptors. Bio-chem. Biophys. Res. Commun. 218(1), 171–175 (1996).
- Kanetsky, P.A., Swoyer, J., Panossian, S., Holmes, R., Guerry, D. and Rebbeck, T.R. A polymorphism in the agouti signaling protein gene is associated with human pigmen-tation. Am. J. Hum. Genet. 70, 770–775 (2002).
- Haigis, M.C. and Sinclair, D.A. Mammalian sirtuins: biological insights and disease rele-vance. Annu. Rev. Pathol. 5, 253–295 (2010).
- Sommer, M., Poliak, N., Upadhyay, S., Ratovitski, E., Nelkin, B.D., Donehower, L.A. and Sidransky, D. DeltaNp63alpha overex-pression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle 5(17), 2005–2011 (2006).
- Ubezio, P. and Civoli, F. Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 16, 509–516 (1994).
- Royall, J.A. and Ischiropoulos, H. Evaluation of 2’,7’-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 302(2), 348–355 (1993).
- Takeo, M., Lee, W., Rabbani, P. et al. EdnrB Governs Regenerative Response of Melano-cyte Stem Cells by Crosstalk with Wnt Sig-naling. Cell Rep. 15(6), 1291–1302 (2016).
- Han, M.N., Lu, J.M., Zhang, G.Y., Yu, J. and Zhao, R.H. Mechanistic Studies on the Use of Polygonum multiflorum for the Treatment of Hair Graying. Biomed. Res. Int. 2015, 651048 (2015).
- Furumura, M., Sakai, C., Potterf, B., Vieira, W. D., Barsh, G.S. and Hearing, V.J. Characterization of genes modulated during pheomelanogenesis using differential display. PNAS 95, 73374–77378 (1998).
- Seiberg, M. Age-induced hair greying – the multiple effects of oxidative stress. Int. J. Cosmet. Sci. 35, 532–538 (2013).
- Commo, S., Wakamatsu, K., Lozano, I., Panhard, S., Loussouarn, G., Bernard, B.A. and Ito, S. Age-dependent changes in eumelanin composition in hairs of various ethnic origins. Int. J. Cosmet. Sci. 34(1), 102–107 (2012).
- Commo, S., Gaillard, O., Thibaut, S. and Bernard, B.A. Absence of TRP-2 in Melanogenic Melanocytes of Human Hair. Pigment Cell Res. 17(5), 488–497 (2004).
- Schwahn, D.J., Timchenko, N.A., Shibahara, S. and Medrano, E.E. Dynamic regulation of the human dopachrome tautomerase promoter by MITF, ERalpha and chromatin remodelers during proliferation and senescence of human melanocytes. Pigment Cell Res. 18, 203–213 (2005).
- Michard, Q., Commo, S., Belaidi, J-P. et al. TRP-2 specifically decreases WM35 cell sensitivity to oxidative stress. Free Radic. Biol. Med. 44, 1023–1031 (2008).
- Thibaut, S., De Becker, E., Caisey, L. et al. Human eyelash characterization. Br. J. Dermatol. 162(2), 304–310 (2010).
- Botchkareva, N.V., Khlgatian, M., Longley, B. J., Botchkarev, V.A. and Gilchrest, B.A. SCF/c-kit signaling is required for cyclic regeneration of the hair pigmentation unit. FASEB J. 15(3), 645–658 (2001).
- Kunisada, T., Yamazaki, H., Hirobe, T. et al. Keratinocyte expression of transgenic hepatocyte growth factor affects melanocyte development, leading to dermal melanocytosis. Mech. Dev. 94(1–2), 67–78 (2000).
- Endou, M., Aoki, H., Kobayashi, T. and Kunisada, T. Prevention of hair graying by factors that promote the growth and differentiation of melanocytes. J. Dermatol. 41(8), 716–723 (2014).
- Sung, J. The use of formulations containing Progenic hair regrowth treatment- Reversing hair graying by activating melanocyte stem cells of hair follicles with platelet-derived growth factor (PDGF). 3rd International Conference and Exhibition on Cosmetology & Trichology July 21-23, Las Vegas, USA (2014).
- Kim, E.J., Park, H.Y., Yaar, M. and Gilchrest, B.A. Modulation of vascular endothelial growth factor receptors in melanocytes. Exp. Dermatol. 14(8), 625–633 (2005).
- Zhai, S., Yaar, M., Doyle, S.M. and Gilchrest, B.A. Nerve growth factor rescues pigment cells from ultraviolet-induced apoptosis by upregulating BCL-2 levels. Exp. Cell Res. 224(2), 335–343 (1996).
- Moreau, M., Neveu, M., Stephan, S. et al. Enhancing cell longevity for cosmetic application: a complementary approach. J. Drugs Dermatol. 6(6 Suppl), s14–s19 (2007).
- Cao, C., Lu, S., Kivlin, R. et al. SIRT1 con-fers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell Mol. Med. 13(9B), 3632–3643 (2009).
- Ohanna, Micka€el, Bonet, Caroline, Bille, Karine et al. SIRT1 promotes proliferation and inhibits the senescence-like phenotype in human melanoma cells. Oncotarget 5(8), 2085–2095 (2014).