Abstract
INTRODUCTION
Hyperpigmentation is a body mechanism that aims to prevent skin tissue damage, including the underlying tissue due to exposure to UV rays. The body’s protective mechanism as a protector against UV rays is to form melanin. Hyperpigmentation conditions will cause various impacts such as lack of confidence and decreased work productivity. The current gold standard of hyperpigmentation treatment is 4% hydroquinone, but it can cause ochronosis effects. Therefore, it is necessary to develop natural ingredients to suppress the side effects of treatment. Kakadu plum (Terminalia ferdinandiana) is a plant that can hide hyperpigmentation.
Kakadu plum (Terminalia ferdinandiana) is a plant from Australia. It contains ascorbic acid of 173.5-322.2mg/g DW, total phenolic of 376.1-505.2 mg GA E/g DW, and ellagic acid of 3,050- 14,020 mg/100 g DW, which has potential effect as anti-hyperpigmentation. High antioxidants in Kakadu plum (Terminalia ferdinandiana) will neutralize free radicals due to UV exposure, and Vitamin C can reduce the amount of melanin in the skin through suppression of anti-tyrosinase activity. Until now, studies measuring the effectiveness of Kakadu plum (Terminalia ferdinandiana) in vivo as an anti-hyperpigmentation agent on male guinea pig skin have not been conducted. Therefore, this study aims to measure Kakadu plum cream’s effectiveness applied to male guinea pig’s skin exposed to UVB, related to its role as an anti-hyperpigmentation agent.
METHODS
This study’s independent variables were 0.1% Kakadu cream and placebo cream; the dependent variables were tyrosinase expression and the amount of melanin. Control variables were age, guinea pig strain, color, genetics, guinea pig feed, guinea pig activity, guinea pig body weight, guinea pig health, and the condition variable is UVB exposure. The Kakadu cream used was 0.1% DBI Kakadu cream produced by PT Derma Beauty Indonesia. In the treatment group, 0.2 mg of Kakadu cream was applied before UVB exposure on the backs of shaved guinea pigs, measuring 2x2cm, for 20 minutes and once a day with each exposure. Male guinea pigs were exposed to UVB rays at a dose of 65 mJ/cm² 3 times a week for 65 seconds, then given Kakadu cream again for 4 hours after exposure. On days without exposure, Kakadu cream is given once a day. In the control group, guinea pigs were given 0.2 mg placebo cream with the same frequency and procedure as the treatment group.
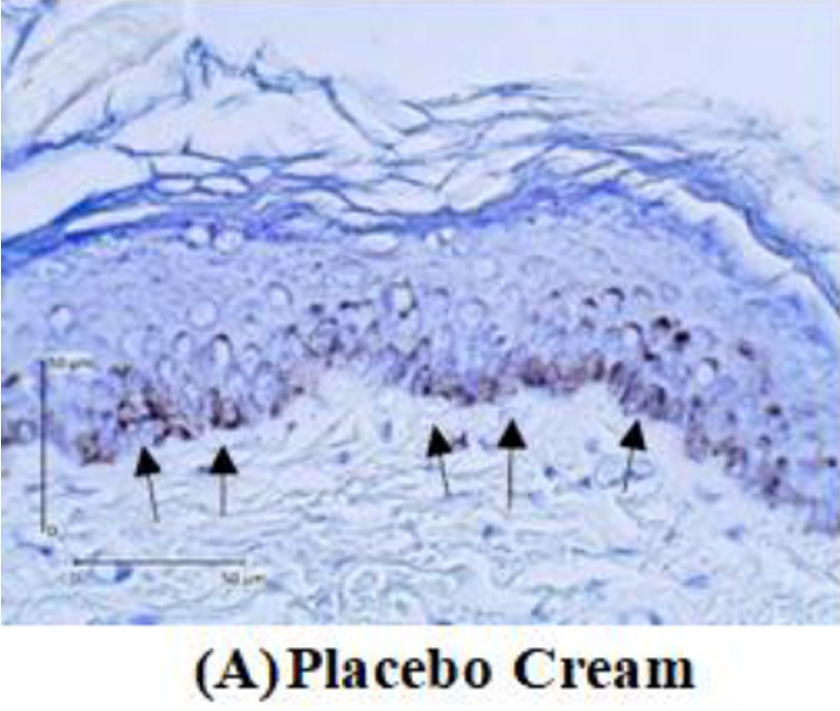
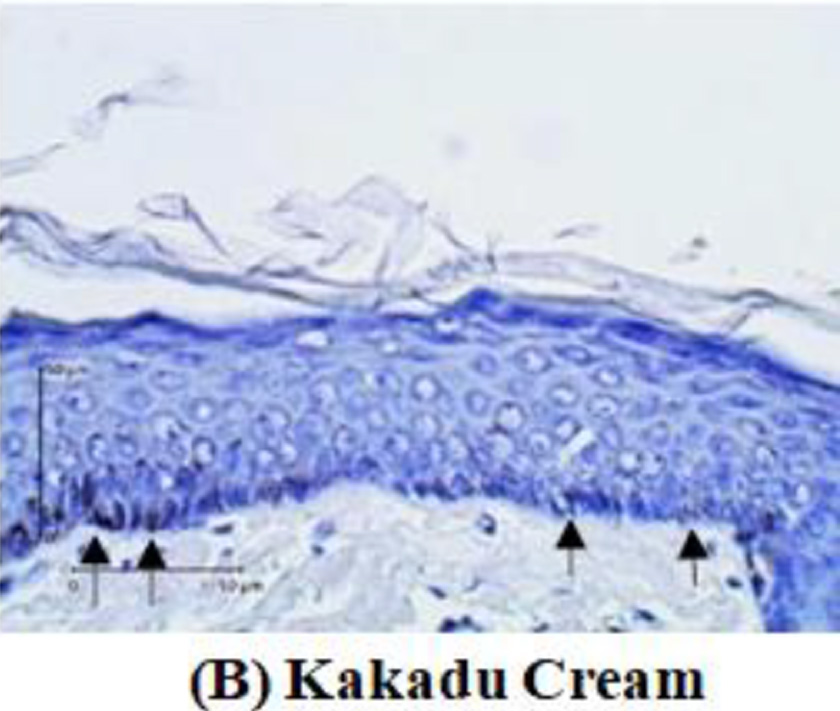
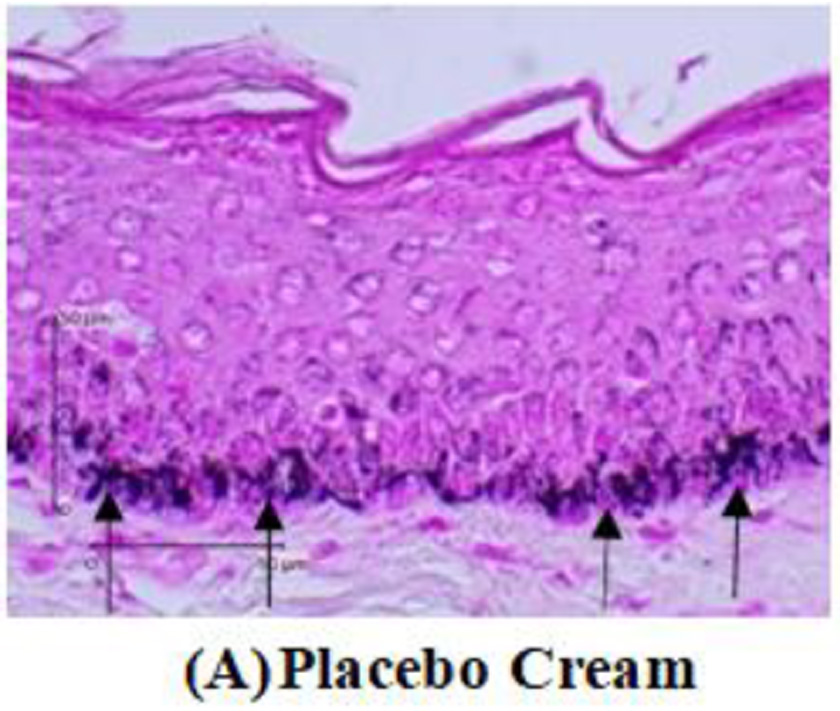
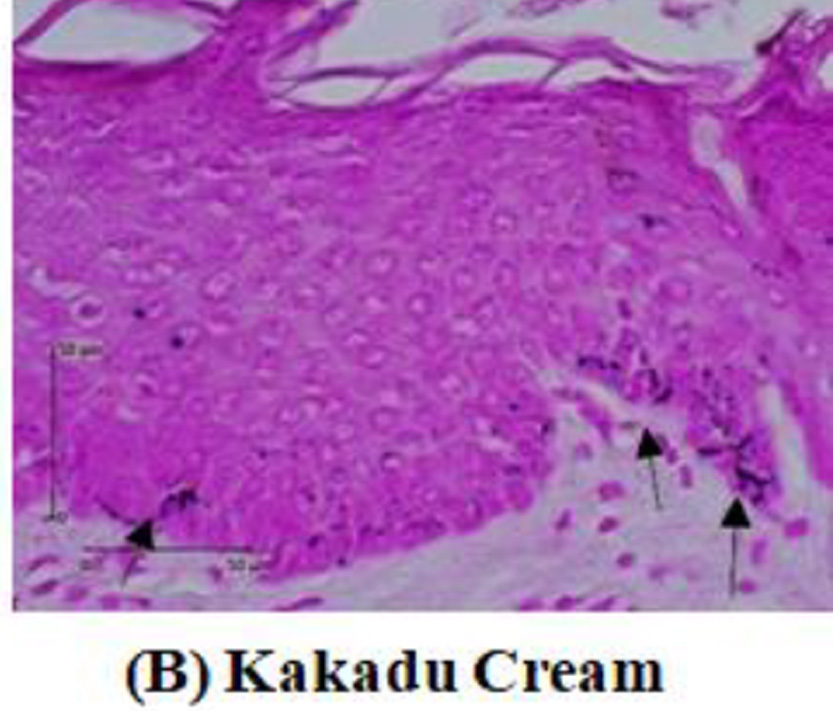
Tyrosinase expression was measured by melanocyte expression in the epidermis using DAKO Envision’s anti-tyrosinase primary antibody kit. Tyrosinase enzyme is indicated by the nucleus melanocyte cells that are blue with brown cytoplasm. The tyrosinase expression will be measured by calculating the tyrosinase pixel divided by the epidermal pixel multiplied by 100%. Meanwhile, the amount of melanin will be measured through a tissue biopsy stained with Masson-Fontana. The calculation is done by comparing the amount of melanin seen in pixels with the entire epidermal tissue visible in pixels multiplied by 100%.
All data collected was tested for data normality with Saphiro Wilk and homogeneity test using Levene’s test. Furthermore, descriptive analysis and comparative analysis will be carried out using parametric statistical tests with an independent sample t-test because the data is normally distributed (p>0.05).
RESULTS
FIGURE 1
FIGURE 2
After two weeks of treated guinea pigs, a tissue biopsy was performed from the guinea pig’s back skin. Histopathological examination was carried out to assess tyrosinase expression by immunohistochemistry analysis. Tyrosinase expression is shown in Figure 2
FIGURE 3
TABLE 1
| Variable | Group | Mem | SD | Median | Min. | Max. |
|
Tyrosinase(%) Melanin (%) |
Placebo Cream 0.1% Kakadu Cream Placebo Cream 0.1% Kakadu Cream |
36.19 14.99 25.93 4.40 |
4.60 2.30 4.52 2.50 |
36.80 14.65 25.90 4.30 |
28.50 10.50 18.70 0.80 |
43.80 19.30 34.50 9.10 |
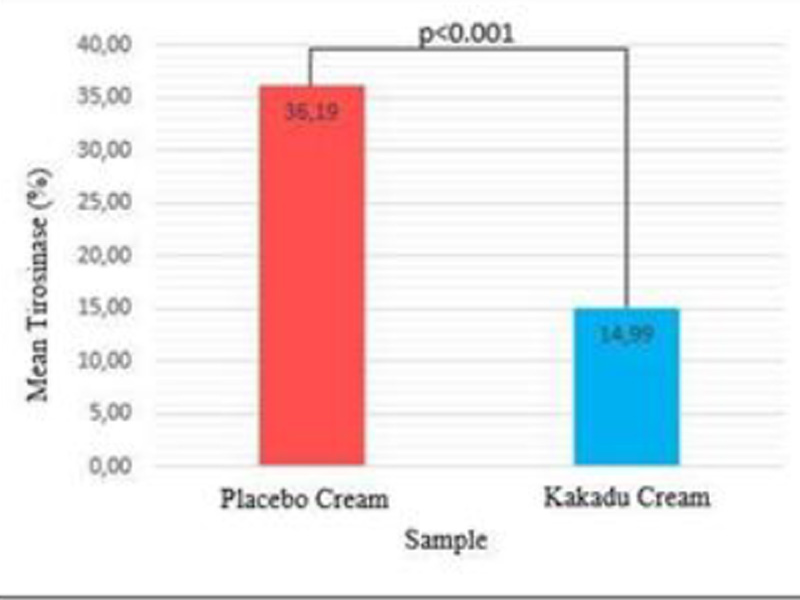
| Variable | Group | Mean±SD | P |
| Tyrosinase(%) | Placebo Cream 0.1% Kakadu Cream | 36.19±4.60
14.99±2.30 |
<0.001 |
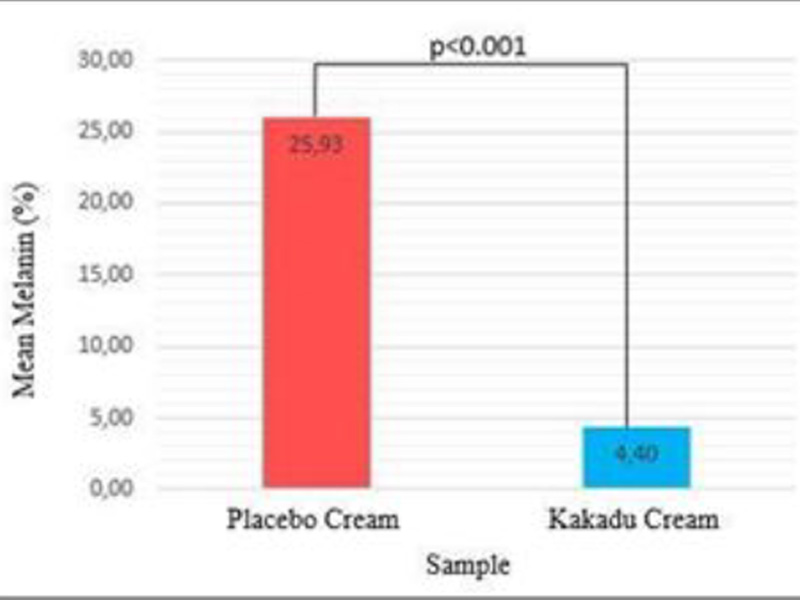
| Melanin (%) | Placebo Cream | 25.93±4.52 | <0.001 |
| 0.1% Kakadu Cream | 4.40±2.50 |
TABLE 2
FIGURE 4
The comparative analysis results showed that the mean amount of melanin in the control group was 25.93±4.52%, while in the treatment group, it was 4.40±2.50%. Comparative analysis using the independent t-test showed that the p<0.001. This indicates that the amount of melanin between the control and treatment groups, after two weeks of treatment is significantly different (p<0.05).
FIGURE 5
Discussion
Research by Panich et al. (2011) compared the inhibitory effect of ascorbic acid and kojic acid on tyrosinase activity, and it was found that kojic acid with a concentration of 240 µM had an inhibitory effect of 30% while ascorbic acid at the same concentration had an inhibitory effect of 70%. Ascorbic acid can stimulate tyrosinase activity, tyrosinase expression and increase the expression of melanogenic regulatory factors, such as tyrosinase-related protein-1 (TRP-1), microphthalmia-associated transcription factor (MITF), and dihydroxy- phenylalanine-chrome-tautomerase (TRP-2). Ascorbic acid also induces mitogen-activated protein kinase (MAPK) phosphorylation. The inhibition of the p38 MAPK pathway by the p38 MAPK inhibitor (10 µM SB203580) will cause suppression of the expression of tyrosinase, TRP-1, and TRP-2 in cells given ascorbic acid.
This study also found a significant difference in the amount of melanin between the control and treatment groups after two treatment weeks. The group was given 0.1% Kakadu cream had a lower amount of melanin than the control group. This is in line with the study by Choi et al. (2009), which compared the amount of melanin in the control group and the treatment group given ascorbic acid to B16 melanoma cells. It was found that the control group had melanin expression of nearly 100%. In contrast, the group was given ascorbic acid of 250.
µM, 500 µM, and 1000 µM had lower amounts of melanin, namely 90%, 60%, and 40%, respectively. The results of a study by Panich et al. (2011) also showed that ascorbic acid with a concentration of 120 µM was able to significantly reduce melanin production in melanoma G361 cells exposed to UVA 16 J/cm².
The anti-melanogenic effect of ascorbic acid is by suppressing reactive oquinones produced by the interaction of tyrosinase and L-DOPA, and by suppressing UV light-mediated oxidant formation so that melanin cannot be formed by tyrosinase action until L-ascorbic acid is oxidized.
UV rays can directly stimulate melanogenesis by suppressing lipids in the plasma membrane of melanocytes so that diacylglycerol is released into the cytoplasm and activating the tyrosinase. UV rays have an indirect effect on melanocytes by inducing keratinocytes to synthesize several paracrine melanocyte factors, for example, basic fibroblast growth factor, endothelin-1, a-melanocyte-stimulating hormone (MSH), and prostaglandin E2 (PGE2), which will stimulate melanocyte proliferation. UV rays can affect the skin by inducing the formation of reactive oxygen species (ROS), which play a role in initiating oxidation reactions during melanogenesis, assisting melanin and inducing melanocyte proliferation.
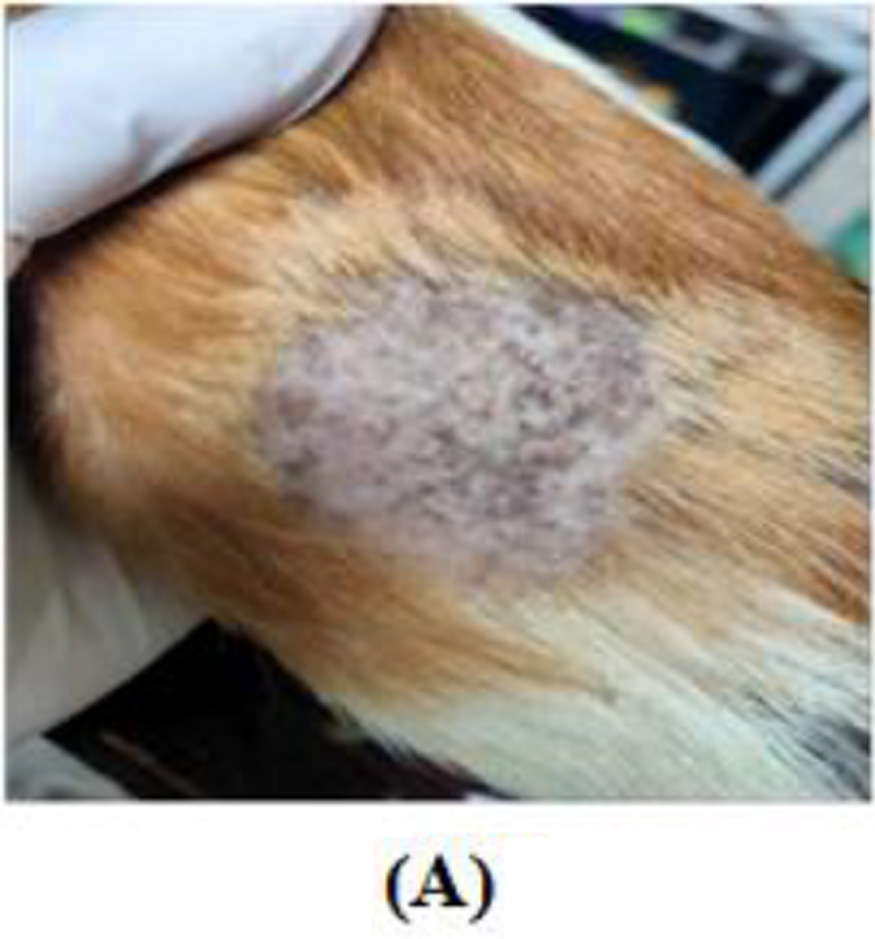
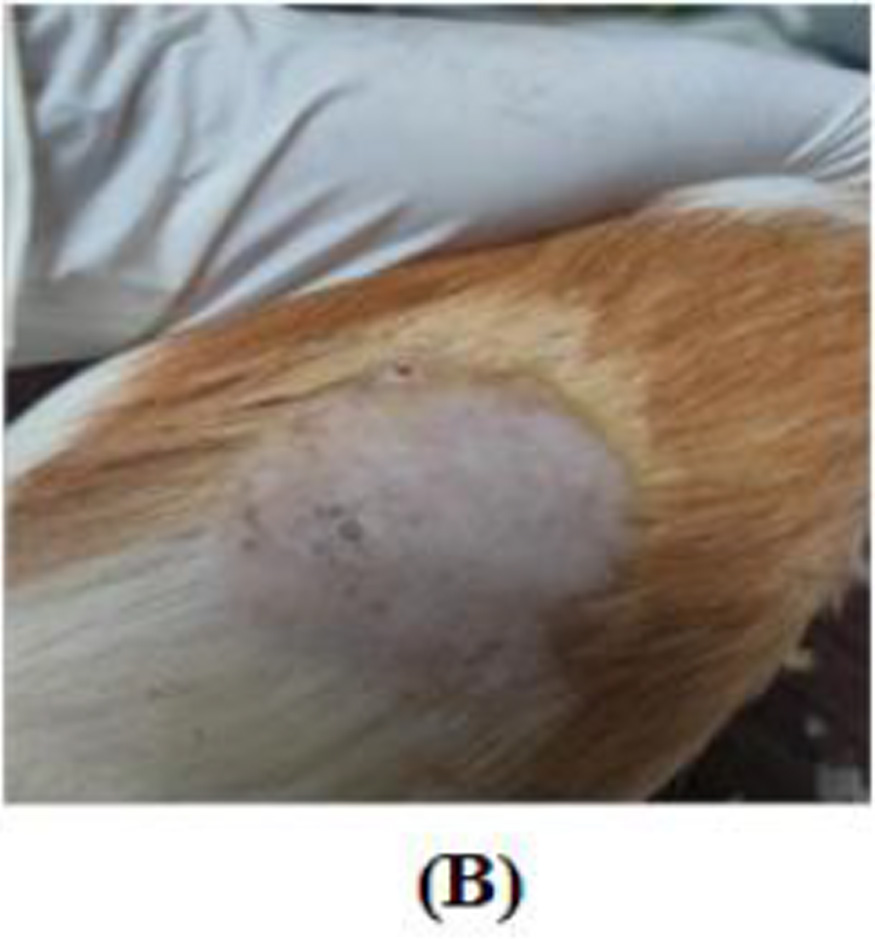
This study shows the effectiveness of Kakadu cream in overcoming hyperpigmentation after UVB exposure. In the treatment group that received 0.1% Kakadu cream, there were fewer brown hyperpigmented lesions. In contrast, there were more hyperpigmented lesions after UVB exposure in the control group applied with a placebo cream. The results of this study are in line with the research of Hwang et al. (2009). That study showed ascorbic acid could have a depigmentation effect by inhibiting the melanogenic peroxidase-catalyzed reaction in melanocytes. Ascorbic acid can change pigmentation from black to brown and indicate significant changes in the skin with melasma.
Kakadu cream also contains ellagic acid, which reduces the expression of IL-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), and UVB-induced TNF-α in vitro. Ellagic acid also helps fibroblasts exposed to UVB to block the secretion of metalloproteinases (MMPs) and cause collagen degradation so that wrinkles on the skin become less. The study by Shimogaki et al. (2000) showed that with a low concentration of 4 µM, ellagic acid could suppress tyrosinase activity by 38.3% and reduce the increase in the amount of melanin by 54.4%. Oral ellagic acid supplementation at a dose of 100-200 mg/day for four weeks can inhibit tyrosinase activity, which is also useful as an anti-melasma.
Several studies have shown the potential of ellagic acid as an antioxidant and anti- inflammatory such as the ability to protect human keratinocyte cells against oxidative stress and UV-induced apoptosis and inhibit the tautomerase activity of Migration Inhibitory Factor (MIF), which is mediated by a pro-inflammatory response.
Besides containing ascorbic acid and ellagic acid, Kakadu plum (Terminalia ferdinandiana) also contains polyphenols and tannins that can suppress tyrosinase expression. According to one study, polyphenols effectively absorb UVB rays in the 290-315 nm spectrum and UVA rays in the 315-400nm spectrum. The inhibitory effect of polyphenols against tyrosinase being the most effective at concentrations of 600 µg/ml. Meanwhile 200 µg/mL tannins reduced intracellular tyrosinase activity and the amount of melanin in melanoma B16 cells, namely 40.3±1.5% and 45.2±1.3%.
Conclusion
Based on this study’s results, it can be concluded that Kakadu plum (Terminalia ferdinandiana) cream 0.1% prevented the tyrosinase expression and the amount of melanin in the ultraviolet B- exposed male guinea pig (Cavia porcellus) skin.
Suggestion
REFERENCES
- Baumann, L., dan Saghari S. Photoaging. 2nd ed.(Baumann, L., Saghari, S., Weisberg E, ed.). New York: McGraw Hill.; 2009.
- Pandel R, Poljšak B, Godic A, Dahmane R. Skin Photoaging and the Role of Antioxidants in Its Prevention. ISRN Dermatol. 2013; 2013:1-11. doi:10.1155/2013/930164.
- Hadiyati P, Sebero H, Apriliana E. Kualitas Hidup pada Pasien Melasma di RSUD Dr.
- H. Abdul Moeloek Lampung. J Kedokt Univ Lampung. 2014; 3 (5):130-138.
- Bruce S. Safety and efficacy of a novel multimodality hydroquinone-free skin brightener over six months. J drugs dermatology. 2013; 12 (3):27-31.
- Konczak I, Maillot F, Dalar A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014; 151:248-256. doi:10.1016/j.foodchem.2013.11.049
- Begin-lavallée V, Lacasse I, Loing E, et al. Active reduces wrinkles. 2015; (July):31-35.
- Choi H Il, Park J Il, Kim HJ, Kim DW, Kim SS. A novel L-ascorbic acid and peptide conjugate with increased stability and collagen biosynthesis. BMB Rep. 2009; 42 (11):743-746. doi:10.5483/BMBRep.2009.42.11.743
- Panich U, Tangsupa-A-Nan V, Onkoksoong T, et al. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch Pharm Res. 2011; 34 (5):811-820. doi:10.1007/s12272-011-0515-3
- Lee SA, Son YO, Kook SH, Choi KC, Lee JC. Ascorbic acid increases the activity and synthesis of tyrosinase in B16F10 cells through activation of p38 mitogen-activated protein kinase. Arch Dermatol Res. 2011; 303 (9):669-678. doi:10.1007/s00403-011- 1158-4
- Hwang SW, Oh DJ, Lee D, Kim JW, Park SW. Clinical efficacy of 25% L-Ascorbic acid (C’ensil) in the treatment of melasma. J Cutan Med Surg. 2009; 13 (2):74-81. doi:10.2310/7750.2008.07092
- Brenner M, Hearing JV. The protective role of melanin against UV. Photochem Photobiol. 2008; 84 (3):539-549. doi:10.1111/j.1751-1097.2007.00226.x.The
- Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol. 2010; 19 (8):182-190. doi:10.1111/j.1600-0625.2009.01044.x
- Shimogaki H, Tanaka Y, Tamai H, Masuda M. In vitro and in vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000; 22 (4):291-303, doi:10.1046/j.1467-2494.2000.00023.x
- Ertam I, Mutlu B, Unal I, Alper S, Kivçak B, Ozer O. Efficiency of ellagic acid and arbutin in melasma: A randomized, prospective, open-label study. J Dermatol. 2008; 35 (9):570-574. doi:10.1111/j.1346-8138.2008.00522.x
- Hseu YC, Chou CW, Senthil Kumar KJ, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012; 50 (5):1245-1255. doi:10.1016/j.fct.2012.02.020
- Sarkar S, Siddiqui AA, Mazumder S, et al. Ellagic Acid, a Dietary Polyphenol, Inhibits Tautomerase Activity of Human Macrophage Migration Inhibitory Factor and Its Pro- inflammatory Responses in Human Peripheral Blood Mononuclear Cells. J Agric Food Chem. 2015; 63 (20):4988-4998. doi:10.1021/acs.jafc.5b00921
- Jesumani V, Du H, Pei P, Aslam M, Huang N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS One. 2020; 15 (1):1-17. doi:10.1371/journal.pone.0227308
- Chai WM, Huang Q, Lin MZ, et al. Condensed Tannins from Longan Bark as Inhibitor of Tyrosinase: Structure, Activity, and Mechanism. J Agric Food Chem. 2018; 66 (4):908-917. doi:10.1021/acs.jafc.7b05481