Abstract
INTRODUCTION
Intracellular lipids such as sphingolipids are considered to play an essential role in the maintenance of water–reten-tion capacity in the stratum corneum2). Ceramides, the key intermediates in the biosynthesis of sphingolipids, have been received increasing attention as an effective moisturizing ingredient for cosmetics and pharmaceuticals, because of the potential water–retention properties. How-ever, the quantity of natural ceramides in organisms is very limited, and thus the large–scale preparation of the lipids from natural resources seems considerably difficult.
Synthetic ceramides are obtained by acylation of the amine group of sphinganine or its derivative, and widely used as moisturizers alternative to natural ones. However, these synthetic approaches for the large–scale preparation of isomerically pure ceramides are tedious and time consuming for commercial applications3). It is thus of great interest to develop a novel and cost–effective natural moisturizer alternative to ceramides.
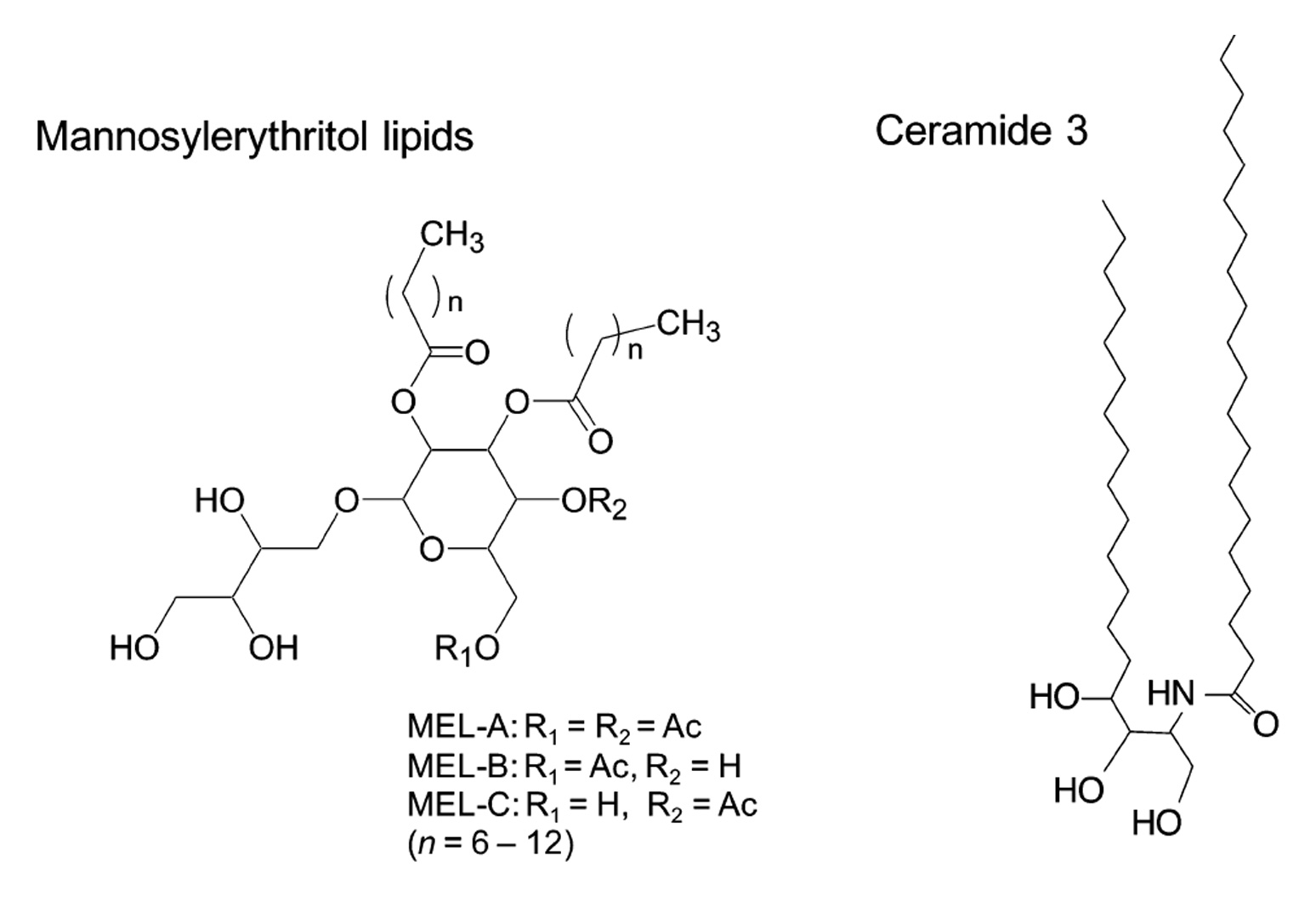
Mannosylerythritol lipids (MELs, Fig. 1) are promising glycolipid biosurfactants, and are abundantly produced at a yield over 100 g/L from vegetable oils by different yeast strains of the genus Pseudozyma4,5). MELs efficiently form different lyotropic liquid crystalline phases including a lamellar (La) in a broad range of concentration6–8). In addition, MEL–A, the major component of MELs, has the similar amphiphilic structure to that of ceramide–3 (Fig. 1) and shows the similar biochemical actions to those of glycosph-ingolipids4). These facts imply that MEL–A would exhibit ceramide–like skin care properties. We thus focused our attention on the feasible use of MEL–A as a moisturizer instead of ceramides and their derivatives.
Novel test methods using no animals have been desired for investigating the skin care properties of cosmetic mate-rials. Recently, living skin equivalent, which consists of three layers of human skin cells (the stratum corneum, epi-dermal, and dermal cells) in a three–dimensional matrix, has been developed as a practical human skin model9,10). In this study, we thus employed a commercially available three–dimensional cultured human skin model for evaluating the moisturizing activity of MEL–A.
Here, we report for the first time the recovery effect of MEL–A on the SDS–damaged human skin cells, and demonstrated that the present yeast biosurfactant has a great potential as a novel and cost–effective moisturizer.
EXPERIMENTAL
2.1 Production of glycolipid, MEL-A
Pseudozyma antarctica T–34 was cultured in a growth medium [4% glucose, 0.3% NaNO3 , 0.03% MgSO4, 0.03%KH2PO4, 0.1% yeast extract (pH 6.0)] at 25˚C on a reciprocal shaker (150 strokes/min) for 2 d11). The obtained seed culture (0.1 mL) was transferred to a 200–mL Erlenmeyer flask containing 20 mL of a production medium [5% soy-bean oil, 0.3% NaNO3, 0.03% MgSO4 , 0.03% KH2PO4, 0.1%yeast extract (pH 6.0)] at 25˚C on a rotary shaker (250 rpm) for 7 d. The produced glycolipids were extracted from the culture medium with an equal amount of ethyl acetate.
2.2 Purification of MEL-A
2.3 Quantification of MEL-A by high-performance liquid chromatography (HPLC)
2.4 Structural analysis
2.5 Viability assay
Fig. 2
Fig. 3
RESULTS AND DISCUSSION
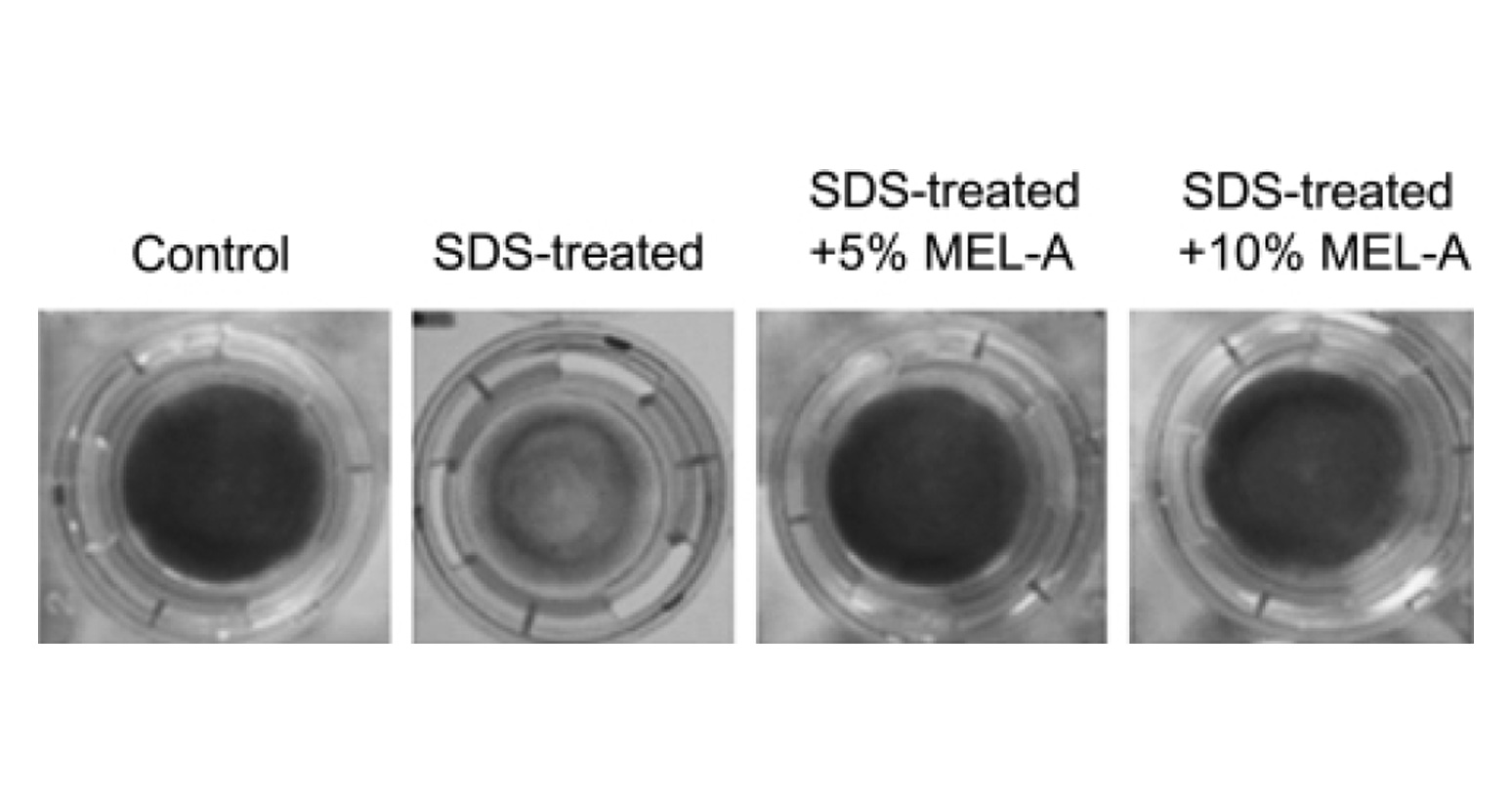
In order to estimate the skin care property of MEL–A, MEL–A was subjected to the cultured human skin model, and the cell viability was determined by the MTT method (Fig. 2). The control cells cultured without the SDS treatment showed a blue color reaction indicating a good viability, whereas the SDS–treated cells hardly showed the color reaction. This means that the SDS treatment clearly lower the cell viability and induce the dry skin condition.
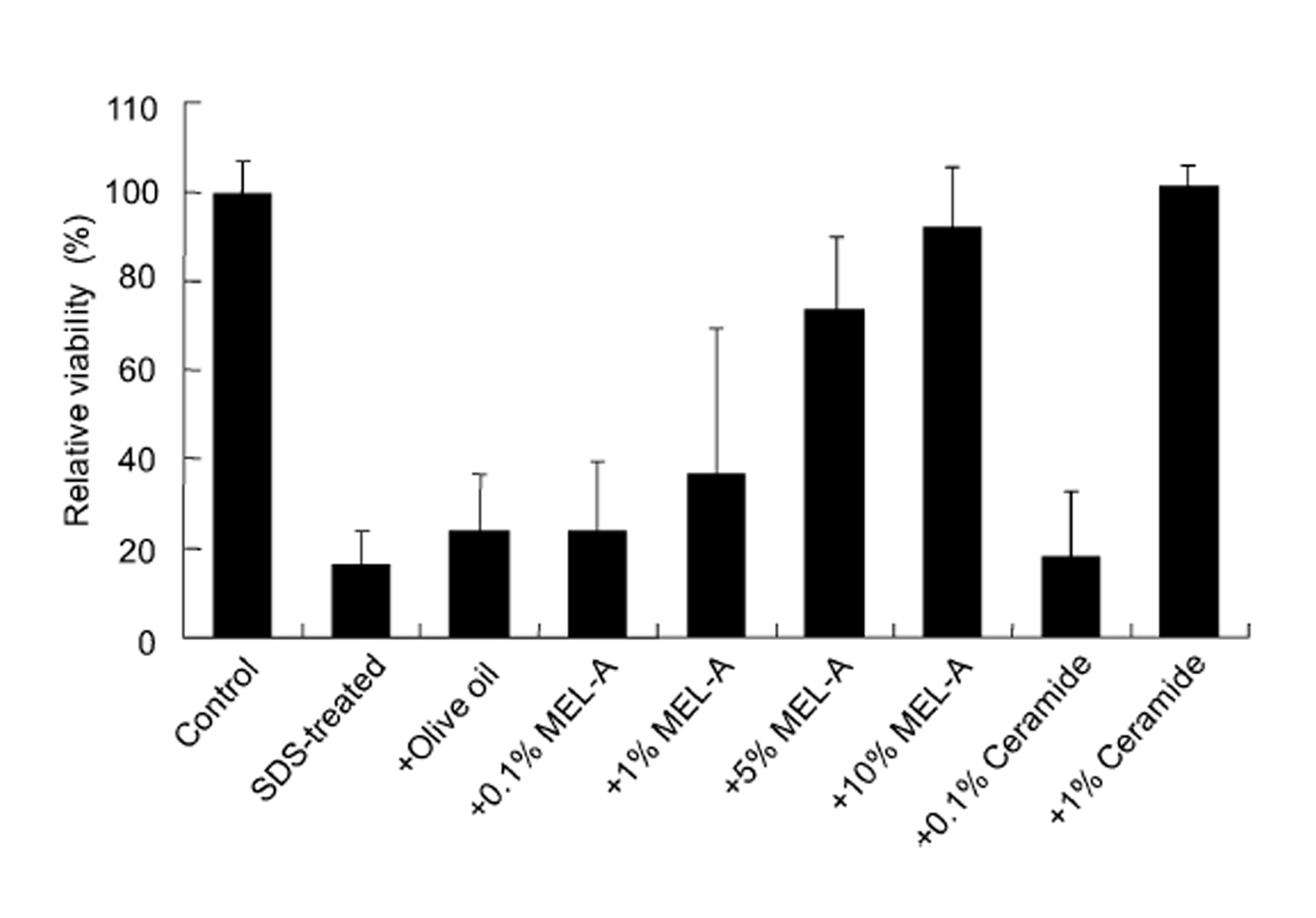
SDS treatment showed the blue color reaction (Fig. 2). The recover y effect of MEL–A on the cells from the SDS–induced damage was observed in a dose–dependent manner. Compared to the control, the cell viability treated with MEL–A solutions of 0.1, 1, 5, 10 wt% were 18.4, 36.2, 73.2, 91.3%, respectively (Fig. 3). Olive oil alone gave little effect on the cell viability after the SDS treatment. In the case of ceramides, the cells treated with 0.1 wt% solution showed little effect on the viability, while the cells with 1 wt% solution completely recovered from the SDS–induced damage. These results suggested that MEL–A has ceramide–like skin care property. The recovery effect of MEL–A toward the SDS–damaged cells is probably due to the moisturizing activity, considering the well–known skin care properties of ceramides. Skin ceramide play an important role in the water permeability properties of the skin, providing an epidermal water barrier which strengthens the skin structure and reduces water loss1-3).
Regarding the mechanism for the recovery effect, we also speculate 1) the structure of MEL–A resembling ceramides would allow the skin cells to be penetrated easily into the intercellular spaces, and 2) the penetrated MEL–A would effectively provide the moisture retention in the cells due to the excellent formation of liquid crystals7).
In conclusion, MEL–A would have a great potential for a novel skin care material, which possesses not only a potential moisturizing activity but also an advantage in the largescale preparation.
ACKNOWLEDGMENTS
REFERENCES
- Lévêque, J.L.; Rigal, J.; Saint–Léger, D.; Billy, D. How does sodium lauryl sulfate alter the skin barrier func-tion in man? A multiparametric approach. Skin phar-macol. 6, 111–115 (1993).
- Imokawa, G.; Akasaki, S.; Minematsu, Y.; Kawai, M. Importance of intracellular lipids in water–retention properties of the stratum corneum: induction and recovery study of surfactant dry skin. Arch. Dermatol. Res. 281, 45–51 (1989).
- Elimelech, R. Process for large–scale preparation of sphingosines and ceramides. US6469148B1 (2002).
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functional and Potential Application of Glycolipid Biosurfactants. J. Biosci. Bioeng. 94, 187–201 (2002).
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Produc-tion of glycolipid biosurfactants by basidiomycetous yeasts. Biotechnol. Appl. Biochem. 53, 39–49 (2009).
- Imura, T.; Yanagishita, H.; Kitamoto, D. Coacervate
- Imura, T.; Ohta, N.; Inoue, K.; Yagi, H.; Negishi, H.; Yanagishita, H.; Kitamoto, D. Naturally engineered gly-colipid biosurfactants leading to distinctive self–assembled structures. Chem. Eur. J. 12, 2434–2440 (2006).
- Imura, T.; Hikosaka, Y.; Worakitkanchanakul, W.; Sakai, H.; Abe, M.; Konishi, M.; Minamikawa, H.; Kitamto, D. Aqueous–phase behavior of natural glycolipid biosur-factants mannosylerythritol lipid A: Sponge, cubic, and lamella phases. Langmuir 23, 1659–1663 (2007).
- Imura, T.; Hikosaka, Y.; Worakitkanchanakul, W.; Sakai, H.; Abe, M.; Konishi, M.; Minamikawa, H.; Kitamto, D. Aqueous–phase behavior of natural glycolipid biosur-factants mannosylerythritol lipid A: Sponge, cubic, and lamella phases. Langmuir 23, 1659–1663 (2007).
- Okeke, C.N.; Tsuboi, R.; Kawai, M.; Yamazaki, M.; Reangchainam, S.; Ogawa, H. Reverse transcription–3′ rapid amplification of cDNA ends–nested PCR of ACT1 and SAP2 mRNA as a means of detecting viable Candi-da albicans in an in vitro cutaneous candidiasis model. J. Invest. Dermatol. 114, 95–100 (2000).
- Sugibayashi, K.; Hayashi, T.; Matsumoto, K.; Hasegawa, T. Utility of a three–dimensional cultured human skin model as a tool to evaluate the simultane-ous diffusion and metabolism of ethyl nicotinate in skin. Drug Metab. Pharmacokinet. 19, 352–362 (2004).
- Kitamoto, D.; Haneishi, K.; Nakahara, T.; Tabuchi, T. Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric. Biol. Chem. 54, 37–40 (1990).
- Morita, T.; Konishi, M.; Fukuoka, T.; Imura, T.; Kitamo-to, D. Discovery of Pseudozyma rugulosa NBRC 10877 as a novel producer of the glycolipid biosurfactants, mannosylerythritol lipids, based on rDNA sequence. Appl. Microbiol. Biotechnol. 73, 305–315 (2006).
- Rau, U.; Nguyen, L.A.; Schulz, S.; Wary, V.; Nimtz, M.; Roeper, H.; Koch, H.; Lang, S. Formation and analysis of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl. Microbiol. Biotechnol. 66, 551–559 (2005).
- Morita, T.; Konishi, M.; Fukuoka, T.; Imura, T.; Kitamo-to, H.K.; Kitamoto, D. Characterization of the genus Pseudozyma by the formation of glycolipid biosurfac-tants, mannosylerythritol lipids. FEMS Yeast Res. 7, 286–292 (2007).