Abstract
Background: Collagen is the primary component in human skin. With age, there is
loss of skin elasticity and collagen, resulting in wrinkle formation and reduction in
skin appearance.
Aims: The objective of this randomized, triple-blind, placebo-controlled study was to evaluate the safety and efficacy of a hydrolyzed marine collagen (Vinh Wellness Collagen, VWC) on aspects of skin health and quality in women between 45 and 60 years of age.
Patients/Methods: Assessments of skin wrinkles, elasticity, and self-reported appearance were conducted using the VISIA skin analysis system, Cutometer®, and Skin QualityVisual Analogue Scale. Outcomes were assessed at weeks 0 (baseline), 6, and 12.
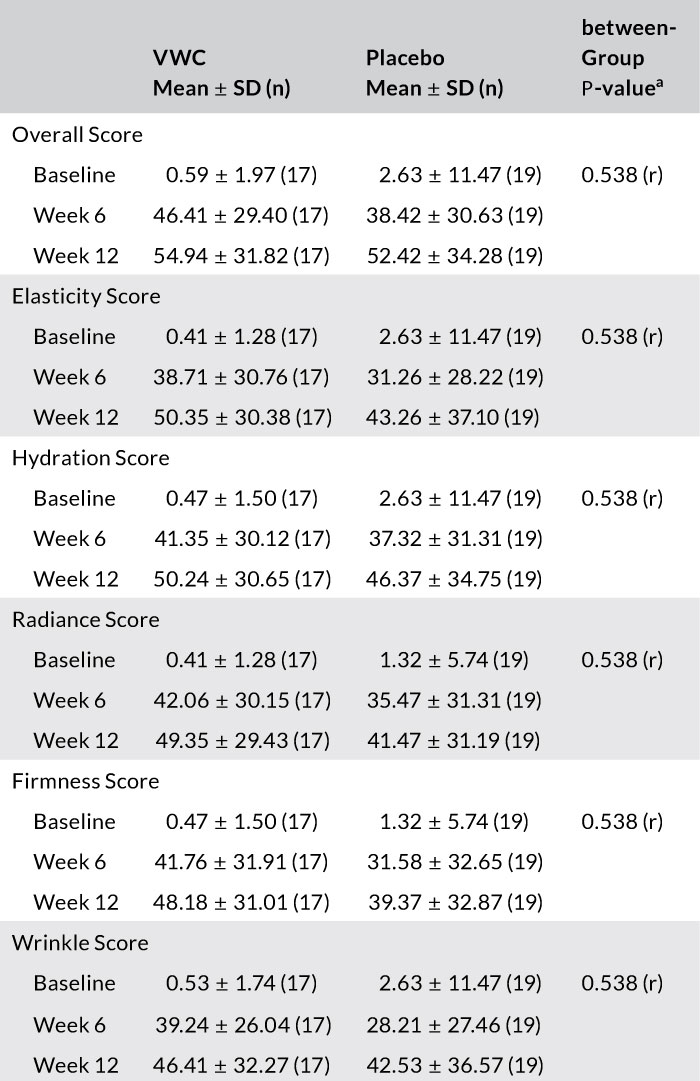
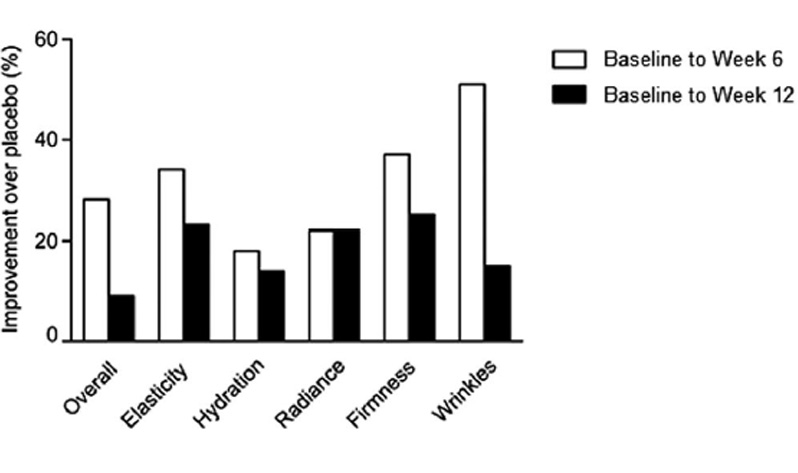
Results: After 12 weeks, participants supplemented with VWC had a significant 35% reduction in wrinkle score (P = .035) from baseline. Participants in the VWC group showed a 24% greater reduction in wrinkles on the right side of the face than those on placebo. A planned subgroup analysis based on age showed women 45-54 years had a significant 20% and 10% improvement in cheek skin elasticity from baseline to week 6 (P = .016) and 12 (P = .022), respectively. At week 12, participants in the VWC group reported greater percentage improvements in overall skin score (9%) and wrinkle (15%), elasticity (23%), hydration (14%), radiance (22%), and firmness (25%) scores vs placebo.
Conclusion: Supplementation with VWC was found to be safe and well-tolerated. The results of this study support the use of fish-derived hydrolyzed collagen for the improvement of skin health in an aging population.
INTRODUCTION
This process slows with age, compromising the quality of collagen fibres.1 Collagen, which constitutes 95% of human skin,2 and elastin are the two primary components of the dermis. These two fibres work synergistically to form the structure of the skin. Aging damages the elastic capacity of the skin, and thus, aging skin is marked by lack of elasticity, fragmentation, and collagen bundle fragility.
Collagen is the most abundant protein in mammals and is currently being promoted by nutrition, biomedicine, and cosmetics in dustries. Gelatin, a protein which is used extensively in the food sector, is a hydrolyzed analog of collagen. Subsequent enzymatic degradation of gelatin results in the generation of hydrolyzed collagen, which contains peptides ranging in molecular weights between <500 Da and 6 KDa, depending on the processing conditions. Orally administered hydrolyzed collagen is absorbed in the small intestine and into the blood stream as peptides and free amino acids and distributed into the dermis for up to 14 days. In the dermis, hydrolyzed collagen provides amino acids for the formation of collagen and elastin fibres, in addition to stimulating endogenous production of new collagen, elastin, and hyaluronic acid.
Hydrolyzed collagen is commonly derived from cow, pig, and chicken sources. In recent years, collagen derived from fish has emerged as an alternative source due to lower environmental impact and risk of disease transmission. Further, marine fish collagen and collagen peptides have a high degree of homology to human structure and bioavailability through the gastrointestinal barrier. Although a number of preclinical studies provide evidence for the beneficial effect of hydrolyzed collagen on skin health, less information is known about the clinical benefits. Previous studies have demonstrated improvements in skin wrinkles, elasticity, and hydration following supplementation with hydrolyzed collagen. Fish collagen peptides have been shown to significantly reduce crow’s feet 14 and periorbital wrinkles in women. Further, hydrolyzed collagen supplementation was reported to improve skin elasticity and moisture while reducing evaporation. The aim of this study was to evaluate the clinical benefits of a 12-week supplementation of a fish-derived collagen peptide on skin wrinkles and elasticity, and self-reported skin appearance.
METHODS
2.1 Participants
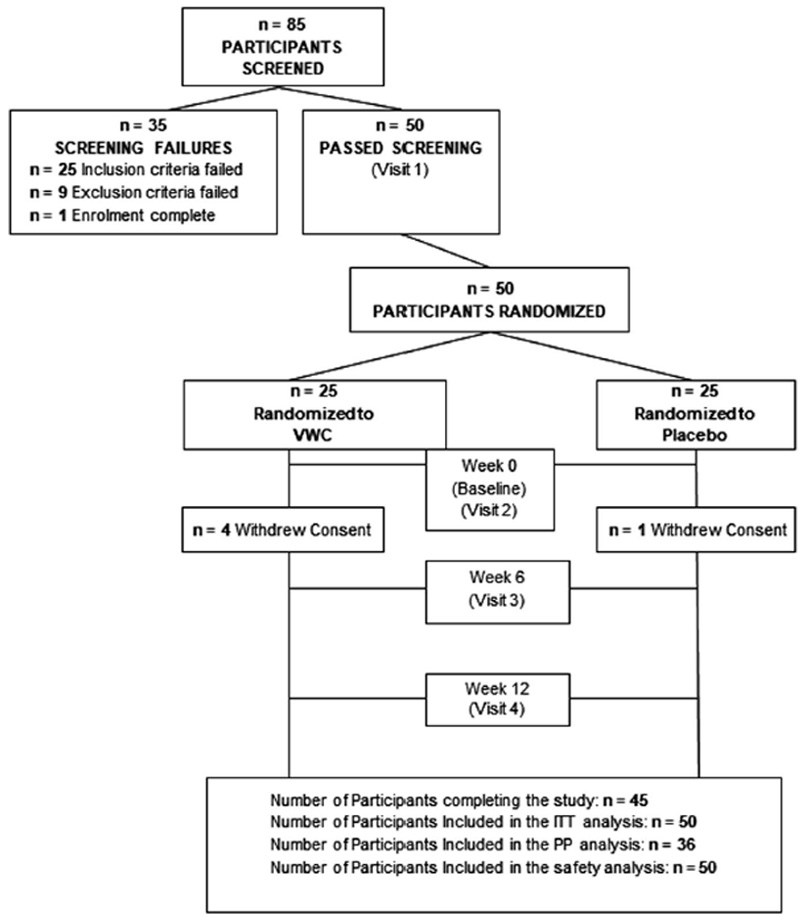
The study design was a randomized, triple-blind, placebo-controlled, parallel study conducted at the KGK Science Inc clinic site (London, Ontario, Canada) from November 25, 2016, to July 18, 2017. Written informed consent was obtained from all participants prior to any study procedures being initiated.
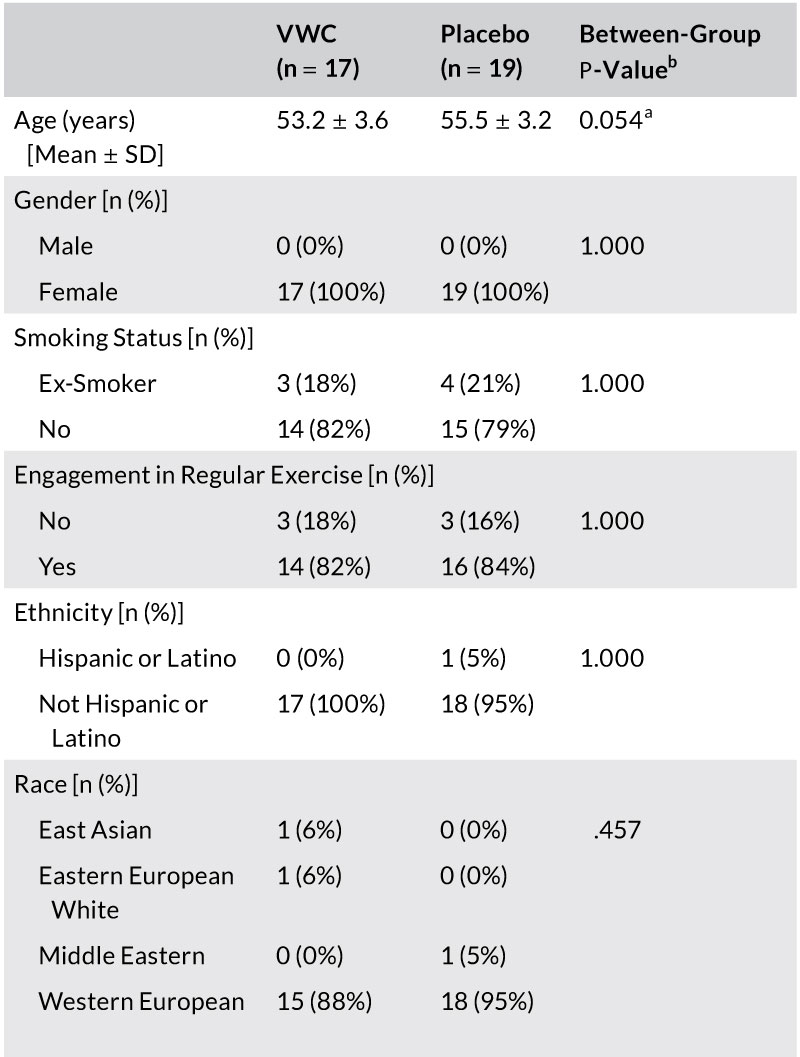
Participants were included if they were females between the ages of 45-60 with a BMI 20.0-29.9 kg/m² and displayed visible signs of natural and photoaging on their face, as assessed by the Fitzpatrick questionnaire at screening. All participants agreed to avoid prolonged exposure to ultraviolet (UV) radiation for the duration of the study.
Individuals were excluded if they suffered from an acute or chronic skin disease or dermatological disorder; used natural health supplements for improving the skin; were on a low protein diet; had planned or unavoidable exposure to UV radiation; had tattoos on or near the test area; used systemic corticosteroids or applied topical alpha hydroxyl acids near the test site within 4 weeks of enrolment; used topical medications near the test area within 6 weeks of enrolment; had Botulinum toxin A (Botox) treatment or filler injection (collagen, hyaluronic acid, etc) near the test sites within 2 years of enrolment; were cognitively impaired and/or unable to give informed consent; or had any other condition which in the medical investigator’s opinion may adversely affect the individual’s ability to complete the study or its measures or which may pose significant risk to the individual.
2.2 Investigational product
2.3 Randomization and blinding
The investigational product and placebo were sealed in sachets that were identical in appearance and labelled per the ICH-GCP requirements and applicable local regulatory guidelines. Unblinded personnel who were not involved in any study assessments labelled the investigational product. The investigators, statistician, other site personnel, and participants were blinded to the products.
2.4 Compliance
2.5 Outcome evaluation
2.6 Skin wrinkle analysis
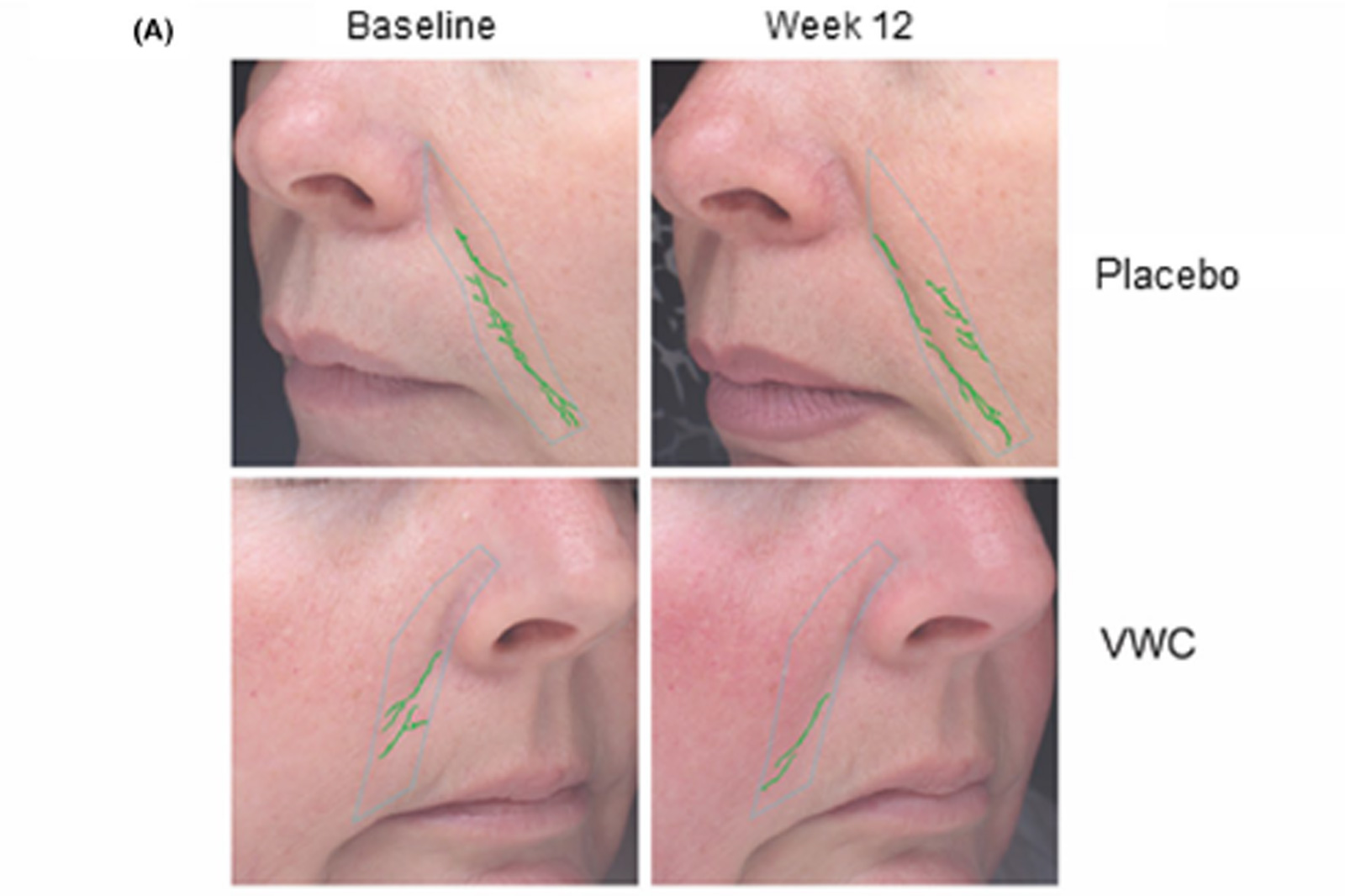
Prior to testing, participants were provided a facial wipe and asked to remove all makeup, after which the face was dried with a lint-free towel or allowed to air dry. Hair was clipped away from the face when applicable. The participant placed their face on the chin (resting) platform of the machine to perform the analysis. Manual masks were created for the nasolabial area of the face of each participant at baseline and were used at each subsequent visit.
The Modified Fitzpatrick Wrinkle Scale (MFWS) is a validated questionnaire evaluating skin wrinkle severity. The MFWS is composed of 3 major classes, in which definitions are based on a set of reference photographs and descriptions, as well as 3 interclasses based only on descriptions. Trained clinic staff applied this scale to participants by using standardized photographs of nasolabial wrinkles alone and assessment of wrinkle depth.
2.7 Skin elasticity analysis
2.8 Skin quality VAS questionnaire
Skin quality was self-assessed by using a Visual Analogue Scale (VAS) questionnaire adapted from Gold et al 2013. Using a scale from 0 (no improvement) to 100 (great improvement), participants were asked to report their skin health based on skin elasticity, hydration, radiance, firmness, wrinkles, and overall feel.
2.9 Laboratory analyses
2.10 Adverse events
2.11 Statistical analyses
The safety population consists of all participants who received any amount of either study product and on whom any postrandomization safety information was available. The per-protocol (PP) population consists of all participants who consumed at least 80% of either study product doses, did not have any major protocol violations, and completed all study visits and procedures connected with measurement of the primary variable. Only observed values were used for the analysis of the PP population. No imputation was performed for missing values of variables.
For continuous endpoints measured on multiple study visits (week 0, week 6, and week 12), descriptive statistics were presented for each study day and for the changes from baseline to each study day. Within-group changes from baseline were assessed using the Wilcoxon signed rank test. Possible differences between groups at baseline were assessed by analysis of variance (ANOVA) with group as a fixed effect. A repeated-measures analysis of covariance (ANCOVA) modeling was employed to assess the changes from baseline between groups. The model included baseline value as a covariate with fixed effects for group, study day, and group by study day interaction. Linear contrast statements from this model were constructed to provide between group p-values at each time point.
A planned subgroup analysis was carried out based on age. This subgroup was created from the PP population, and all analyses were conducted in the same manner as the whole set.
Probabilities ≤.05 were considered statistically significant. All statistical analyses were completed using SAS version 9.3.1 (Cary, NC) for Microsoft Windows.
RESULTS
FIGURE 1
3.1 Efficacy of VWC supplementation on skin quality
TABLE 1
TABLE 2
FIGURE 2
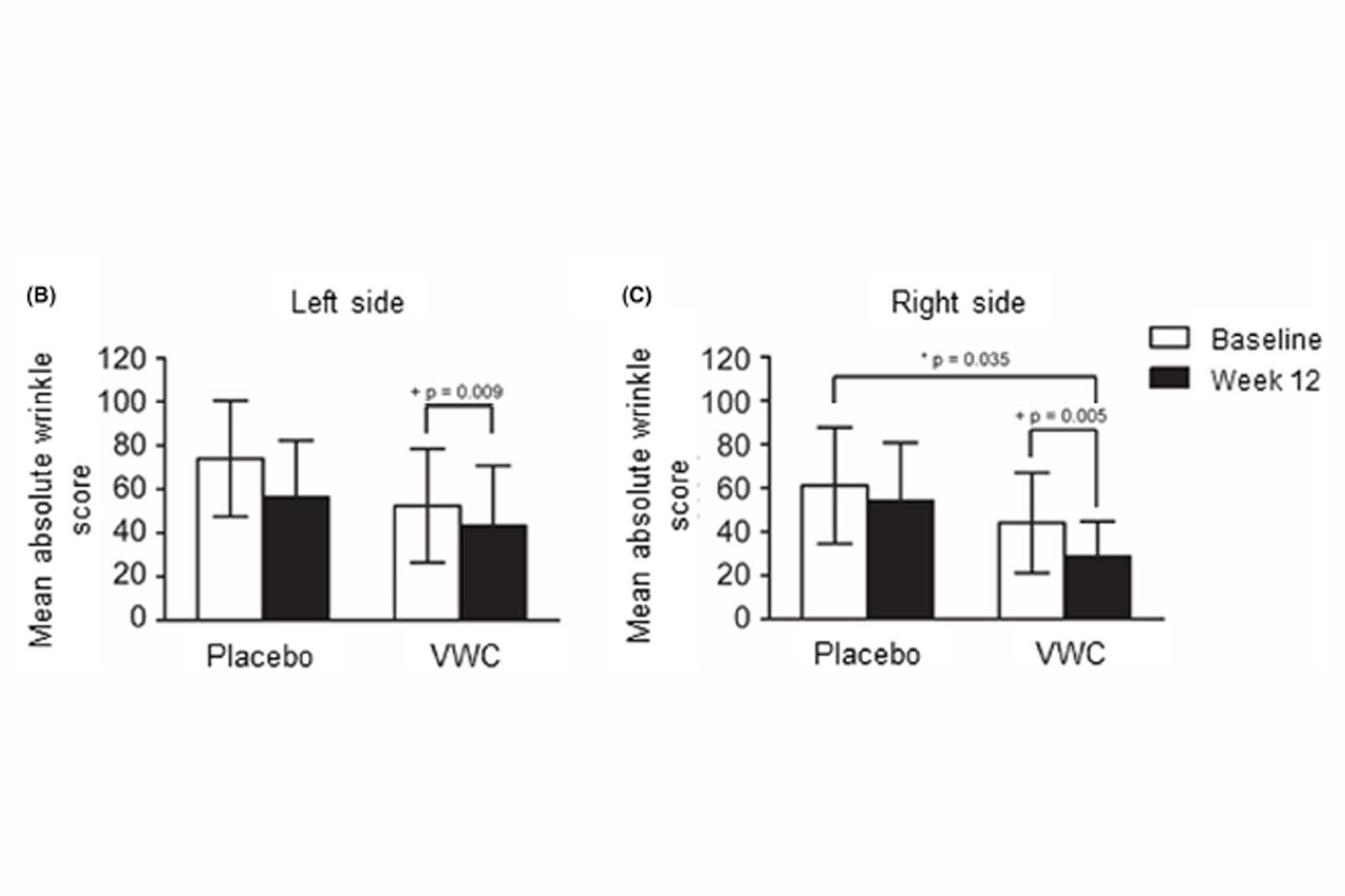
3.2 Efficacy of VWC supplementation on wrinkle count and appearance
(P = .035) (Figure 3C).
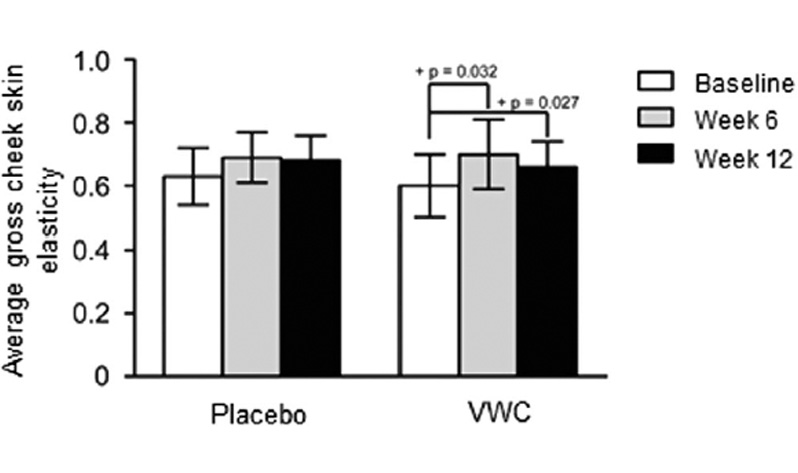
3.3 Efficacy of VWC supplementation on skin elasticity
FIGURE 3
3.4 Safety evaluation of VWC supplementation
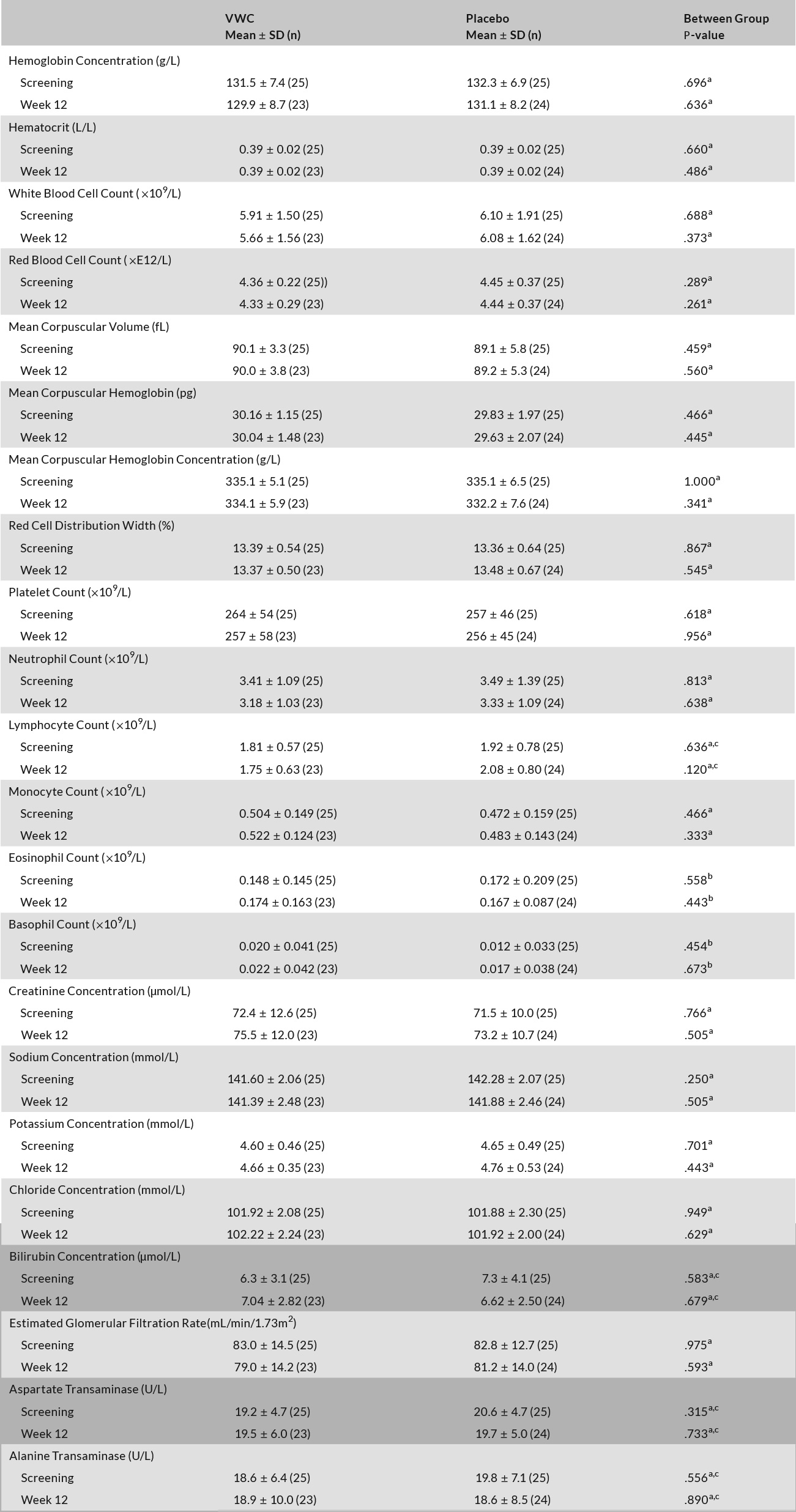
There was no between-group differences in hematological and clinical chemistry parameters at screening or after 12 weeks (Table 3), and all participants were deemed healthy. For both groups, all hematology, clinical chemistry, electrolytes, and liver and kidney function markers remained within healthy clinical reference ranges. Any changes in these safety parameters were deemed not clinically significant. There were no significant differences in vital signs or anthropometric measurements between VWC and placebo (data not shown).
DISCUSSION
There was no between-group differences in hematological and clinical chemistry parameters at screening or after 12 weeks (Table 3), and all participants were deemed healthy. For both groups, all hematology, clinical chemistry, electrolytes, and liver and kidney function markers remained within healthy clinical reference ranges. Any changes in these safety parameters were deemed not clinically significant. There were no significant differences in vital signs or anthropometric measurements between VWC and placebo (data not shown).
FIGURE 4
Gross cheek skin elasticity after supplementation with VWC for participants 45-54 years of age (n = 12), assessed by the Cutometer®. Higher values indicate more elastic skin. + Within group p-values generated by the Wilcoxon signed rank test. Probability values P ≤ .05 are statistically significant supplemented with VWC showed a significant 24% improvement in the absolute wrinkle score on the right side of the face compared to placebo. On both sides of the face, there was a significant decrease in the wrinkle score from baseline to week 12 for participants supplemented with VWC.
The improvements in skin health observed in the current study are consistent with previous studies examining collagen supple-mentation. Supplementation with 3 g of collagen peptide was as-sociated with a higher report of participant satisfaction compared to placebo.13 Similar improvements in facial wrinkles in this study were reported following 30 days of hydrolyzed collagen supplementation in females.18 Bonnet al. (2017) found that following 12-week supplementation with 10 g of fish collagen peptides, women had a significant 10% reduction in periorbital wrinkles.19 As the improvement found by Bonnet et al (2017) was for periorbital wrinkles, a direct comparison to the current study cannot be drawn. However, one possible reason for the greater improvement in wrinkle score observed in the current study may be due to higher concentration of anti-oxidant amino acids (glycine, proline, hydroxyproline) found in VWC.
Although not significant, the improvement in cheek skin elasticity over the 12-week VWC supplementation period is consistent with previously published research that utilized similar doses and study populations. Korean females and males experienced a significant improvement in elasticity following 12-week supplementation with 3 g of collagen peptide and vitamin C 13 or 3 g hydrolyzed fish collagen combined with astaxanthin.20 Supplementation with 10 g of collagen peptides combined with vitamins A, C, and E and zinc significantly improved gross cheek elasticity in females aged 40-60 years after 90 days.21 This suggests collagen peptides may act synergistically with other nutrients to improve skin elasticity. Future studies should consider investigating the potential synergistic effects of VWC with other skin enhancing nutrients.
This is the first study to report a differential response between right and left facial areas with collagen supplementation. As the absolute wrinkle score reflects several factors, both photoaging and melanoma are generally more prevalent on the left side of the face due to sun exposure while driving.22 Sleeping habits causing micro-pressure over an extended time have also been suggested to contribute to aging lines and faster aging on the left side of the face.23 It may be that the left cheek was more photoaged and more affected by sleep lines than the right, making the determination of product efficacy challenging. This is one possible explanation for the significant results between VWC and placebo for the right cheek, but only significant changes from baseline to week 12 for the VWC group for the left. This suggests that longer usage of the product may result in significant differences between VWC and placebo on the left side of the face; however, this warrants further investigation in future studies.
A planned subgroup analysis based on age found that females between 45 and 54 years supplemented with VWC had significant improvements in skin elasticity at week 12. Compared to baseline, these women had 20% and 10% improvements in the cheek skin gross elasticity at weeks 6 and 12, respectively. There were no significant
TABLE 3
A limitation of measurements and specifically re-measurements of any viscoelastic property is the phenomenon of hysteresis. As such, repeated measurements of viscoelastic material are discouraged as they cannot account for the inability of the property (ie, skin) to return to its prestrained state prior to the next measurement cycle and the impression is then carried forward into subsequent measurements. In the current study, 9 Cutometer® measurements for gross elasticity were either out of range or missing. Unfortunately, these measurements could not be repeated without introducing the confounding factor of hysteresis. The test-re-rest reliability of skin elasticity measurements while avoiding cofounding has been described in a previous study.24 Given this information, a potential solution would be to perform baseline measurements of cheek skin elasticity on both cheeks, such that any measurement out of range can be performed on the other cheek without hindering the statistical significance of the results. Recognizing that it may not be possible to use the values from one cheek to replace another, previous studies have included a rest period of 45 minutes between repeated measurements in order to avoid consequences of hyster-esis.24 Further, repeated measurements, up to three times,25 would provide a mean score and potentially accommodate out of range or missing values. Future work should incorporate these methods to reduce missing data.
Abbreviaions: n, number; SD, standard deviation.
Between-group comparisons were made using the independent Student’s t test.
Between-group comparisons were made using the Mann-Whitney U test.
Logarithmic transformation was required to achieve normality.
CONCLUSION
DATA AVAILABILITY STATEMENT
DATA AVAILABILITY STATEMENT
Malkanthi Evans : https://orcid.org/0000-0003-0832-677X
Erin D. Lewis : https://orcid.org/0000-0002-1760-3073
REFERENCES
- Bonta M, Daina L, Mutiu G. The process of ageing reflected by histological changes in the skin. Rom J Morphol Embryol. 2013;54(3 suppl):797-804.
- Gelse K, Poschl E, Aigner T. Collagens–structure, function, and bio-synthesis. Adv Drug Deliv Rev. 2003;55(12):1531-1546.
- Baumann L. Skin ageing and its treatment. J Pathol. 2007;211(2):241-251.
- Calleja-Agius J, Brincat M, Borg M. Skin connective tissue and ageing. Best Pract Res Clin Obstet Gynaecol. 2013;27(5):727-740.
- Skovgaard GRL, Jensen AS, Sigler ML. Effect of a novel dietary supplement on skin aging in post-menopausal women. Eur J Clin Nutr. 2006;60(10):1201-1206.
- Zague V. A new view concerning the effects of collagen hydrolysate intake on skin properties. Arch Dermatol Res. 2008;300(9):479-483.
- Djagny VB, Wang Z, Xu S. Gelatin: a valuable protein for food and pharmaceutical industries: review. Crit Rev Food Sci Nutr. 2001;41(6):481-492.
- Watanabe-Kamiyama M, Shimizu M, Kamiyama S, et al. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J Agricultural Food Chem. 2010;58(2):835-841.
- Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Genovese L. An over-view of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J. 2015;8:29-42.
- De Luca C, Mikhal’chik EV, Suprun MV, Papacharalambous M, Truhanov AI, Korkina LG. Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-de-rived antioxidants: a single-blind case-control clinical study. Oxid Med Cell Longev. 2016;2016:1-14.
- Tanaka M, Yamamoto Y, Misawa E, et al. Effects of aloe sterol sup-plementation on skin elasticity, hydration, and collagen score: a 12-week double-blind, randomized controlled trial. Skin Pharmacol Physiol. 2016;29(6):309-317.
- Proksch E, Schunck M, Zague V, Segger D, Degwert J, Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol Physiol. 2014;27(3):113-119.
- Choi SY, Ko EJ, Lee YH, et al. Effects of collagen tripeptide supplement on skin properties: A prospective, randomized, controlled study. J Cosmet Laser Ther. 2014;16(3):132-137.
- Luc Duteil CQR, Bruno-Bonnet C, Lacour JP. Effect of low dose type I fish collagen peptides combined or not with silicon on skin aging signs in mature women. JOJ Case Stud. 2018;6(4):001–005.
- Koizumi S, Inoue N, Shimizu M, Kwon C-J, Kim H-Y, Park KS. Effects of Dietary Supplementation with Fish Scales-Derived Collagen Peptides on Skin Parameters and Condition: A Randomized, Placebo-Controlled, Double-Blind Study. Int J Peptide Res Ther. 2018;24(3):397-402.
- Sumida E, Hiorata A, Kuwaba K. The Effect of Oral Ingestion of Collagen Peptide on Skin Hydration and Biochemical Data of Blood. J Nutr Food. 2004;7:45-52.
- Gold MH, Biron JA. Safety and Cosmetic Effects of Photodynamic Therapy using Hexyl Aminolevulinate and Intense Pulsed Light: A Pilot Study Conducted in Subjects with Mild-to-moderate Facial Photodamage. J Clin Aesthet Dermatol. 2013;6(10):27-31.
- Zhou S-L, Wang H-Y, Yue D-X. Clinical Effects and Safety of Oral Treatment with Low-Molecular Fish Collagen Hydrolysate on Female Facial Skin Properties. J Pract Dermatol. 2011;4(3).
- Bonnet D.Introduction to Naticol® marine collagen peptides for anti-aging and overview of clinical studies. https://pdfs.semanticscholar.org/fed1/fa27de3676b4626343d35ea1d1567ca3f216.pdf Accessed October 5 2017.
- Yoon HS, Cho HH, Cho S, Lee SR, Shin MH, Chung JH. Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metallo-proteinase-1 and -12 expression: a comparative study with placebo. J Medicinal Food. 2014;17(7):810-816.
- Campos P, Melo M, Calixto L, Fossa M. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo-controlled study. Clinical Pharmacology & Biopharmaceutics. 2015;04(03):1–6.
- Paulson KG, Iyer JG, Nghiem P. Asymmetric lateral distribution of melanoma and Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2011;65(1):35-39.
- Poljsak B, Godic A, Lampe T, Dahmane R. The influence of the sleeping on the formation of facial wrinkles. J Cosmet Laser Ther. 2012;14(3):133-138.
- Peperkamp K, Verhulst AC, Tielemans HJP, Winters H, van Dalen D, Ulrich DJO. The inter-rater and test-retest reliability of skin thick-ness and skin elasticity measurements by the DermaLab Combo in healthy participants. Skin Res Technol. 2019;25(6):787-792.
- Pmc MBG, Meloo M, Calixto L, Fossa M. An oral supplementation based on hydrolyzed collagen and vitamins improves skin elasticity and dermis echogenicity: a clinical placebo-controlled study. Clin Pharmacol Biopharm. 2015;4(142):2.
How to cite this article: Evans M, Lewis ED, Zakaria N, Pelipyagina T, Guthrie N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J Cosmet Dermatol. 2020;00:1–10. https://doi.org/10.1111/jocd.13676